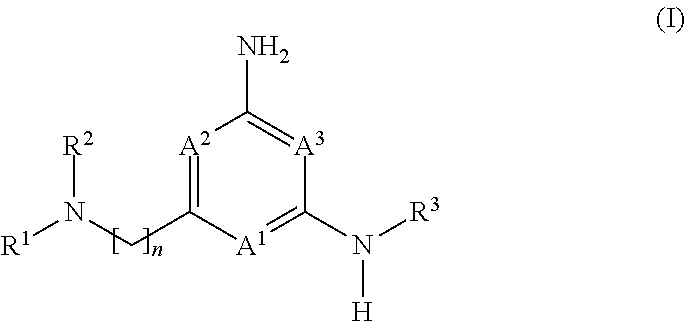
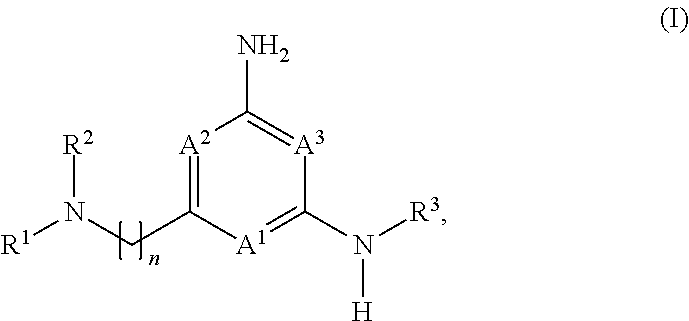
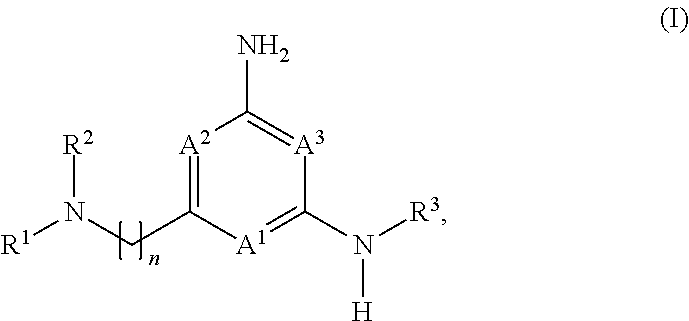
Heteroaryl compounds and their use
a technology of heteroaryl compounds and compounds, applied in the field of heteroaryl compounds, can solve problems such as diarrhea, peripheral nerve damage, side effects,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6-((Methyl(phenyl)amino)methyl)-N2-(p-tolyl)-1,3,5-triazine-2,4-diamine
[0322]
[0323]Yield: 9%.
[0324]HPLC-MS [M+H]+: 321; Rt=2.72 min (Method C).
[0325]1H NMR (400 MHz, CDCl3) δ: 7.28 (s, 2H), 7.15 (ddd, J=7.3, 5.9, 2.3 Hz, 2H), 7.00 (d, J=7.4 Hz, 2H), 6.84 (s, 1H), 6.71 (dd, J=8.8, 0.9 Hz, 2H), 6.65 (td, J=7.3, 1.0 Hz, 1H), 5.04 (s, 2H), 4.26 (s, 2H), 3.07 (s, 3H), 2.24 (s, 3H).
example 2
N2-(4-Methoxyphenyl)-6-((methyl(phenyl)amino)methyl)-1,3,5-triazine-2,4-diamine
[0326]
[0327]Yield: 6%.
[0328]HPLC-MS [M+H]+: 337; Rt=2.43 min (Method C).
[0329]1H NMR (400 MHz, CDCl3) δ: 7.24 (d, J=20.0 Hz, 2H), 7.18-7.13 (m, 2H), 6.79 (s, 1H), 6.75 (s, 2H), 6.72-6.68 (m, 2H), 6.67-6.62 (m, 1H), 5.05 (s, 2H), 4.25 (s, 2H), 3.72 (s, 3H), 3.05 (s, 3H).
example 3
N2-β-Methoxyphenyl)-6-((methyl(phenyl)amino)methyl)-1,3,5-triazine-2,4-diamine
[0330]
[0331]Yield: 21%.
[0332]HPLC-MS [M+H]+: 337; Rt=2.65 min (Method C).
[0333]1H NMR (400 MHz, CDCl3) δ: 7.19 (d, J=2.1 Hz, 1H), 7.17-7.13 (m, 2H), 7.10 (t, J=8.5 Hz, 1H), 6.97 (s, 1H), 6.93 (dd, J=8.2, 1.4 Hz, 1H), 6.69 (dt, J=3.5, 1.8 Hz, 2H), 6.64 (tt, J=5.0, 2.5 Hz, 1H), 6.57-6.51 (m, 1H), 5.16 (s, 2H), 4.26 (s, 2H), 3.70 (s, 3H), 3.07 (s, 3H).
General Procedure III
[0334]
[0335]To a stirred solution of the appropriate biguanide hydrochloride salt (ex: p-tolylbiguanide) (1.0 eq) in methanol (2.4 mL / mmol) was added sodium methoxide (1.0 eq, 25 wt % in methanol) at room temperature and it was stirred for 30 min. The appropriate ester was added to the reaction mixture (ex: ethyl N-benzyl-N-methylglycinate) (1.2 eq) and heated to 70° C. for 18 h. After reaction completion, the reaction mixture was poured into cold water and the organic product was extracted with ethyl acetate. Organic extracts were dried ove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com