Bioresponsive Particles
a technology of bioresponsive particles and bioactive particles, applied in the field of bioresponsive particles, can solve the problems of limiting the ability of the particles to reach the cellular microenvironment in sufficient quantities to adequately fight cancer, and the treatment is completely ineffectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
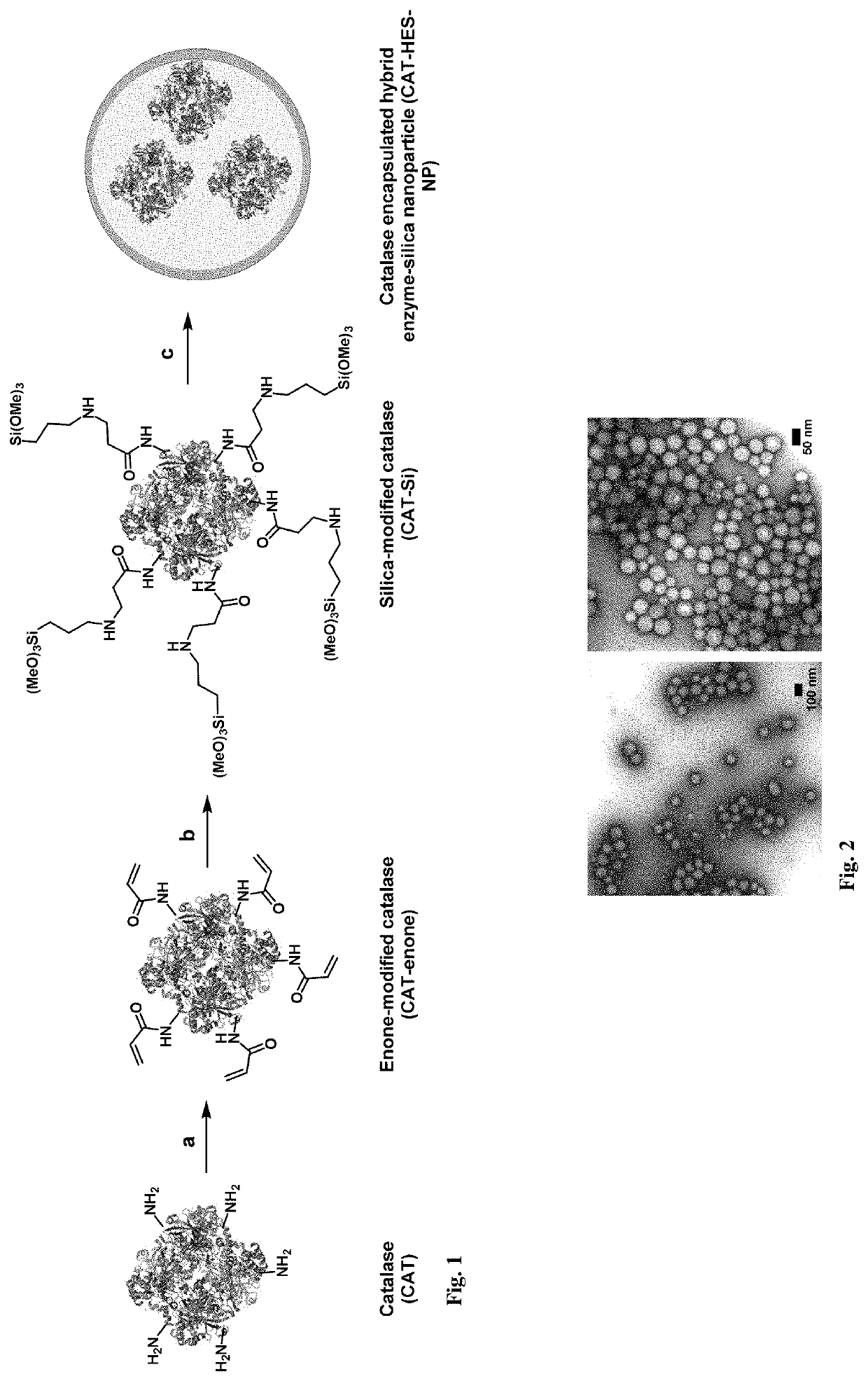
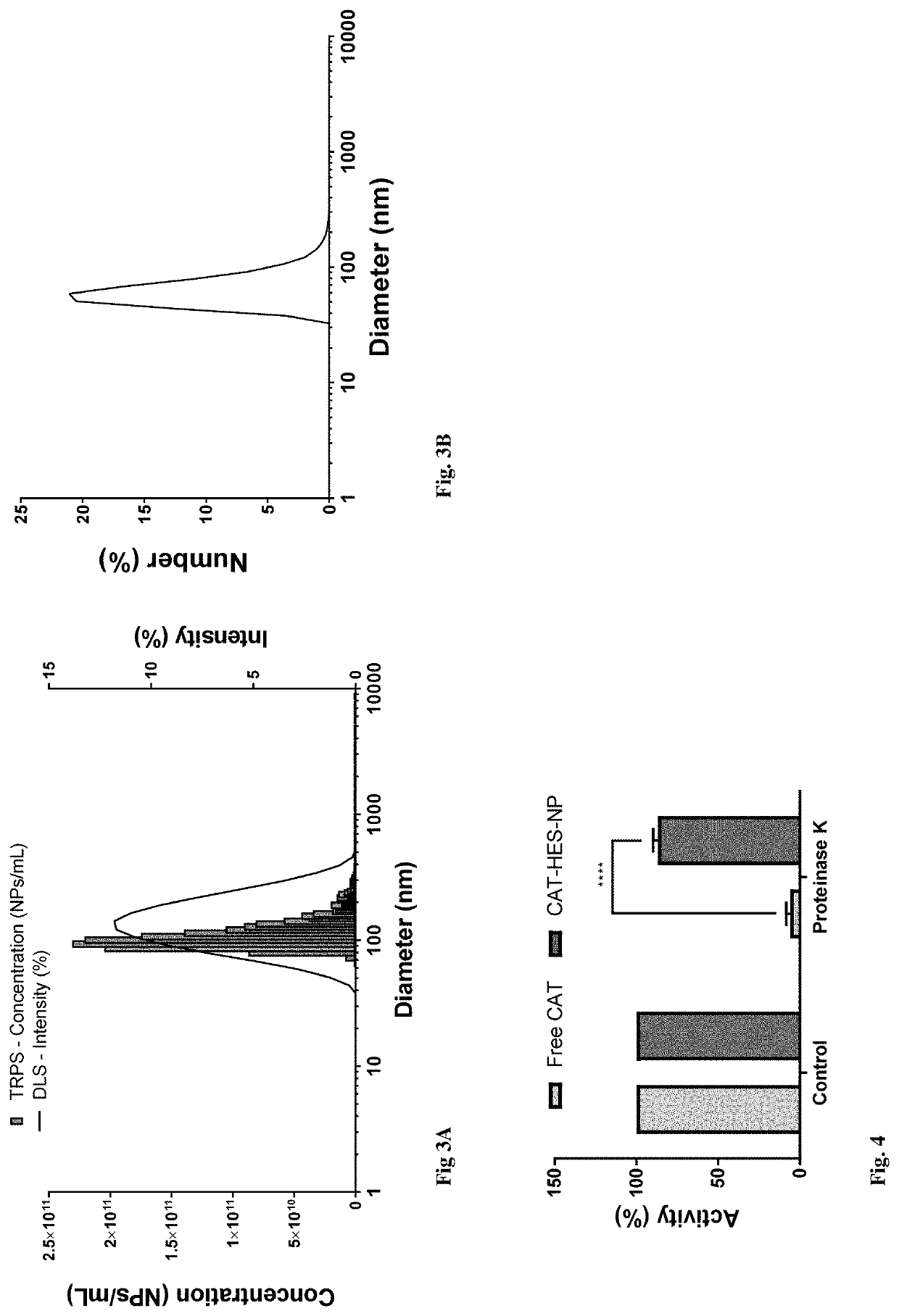
[0036]We disclose a novel hybrid approach to shielding enzymes. We first modify the enzyme surface with silica precursors and then proceed to deposit silica to a desired thickness while retaining its biological activity. An advantage of this approach is that we can control final nanoparticle size and desired enzyme activity per particle by incorporating one or more or different enzyme molecules to optimize delivery and efficacy. Unlike passive trapping of enzymes in hollow silica spheres that utilize templates ≥100 nanometers, our nanoparticles can be made as small as 20-50 nanometer to achieve optimal delivery and enzyme activity. In an embodiment we exemplify the method with catalase as a model enzyme because it can be used to detect tissues in oxidative stress using ultrasound imaging, can be used as an anti-oxidant, and its activity is easily measured using commercial assay kits. In another embodiment example, we used our method to encapsulate catalase and also to encapsulate as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com