Methods and compositions for mobilizing stem cells
a technology of stem cells and compositions, applied in drug compositions, plant/algae/fungi/lichens, immune disorders, etc., can solve the problems of critical unmet needs, and achieve the effect of assessing the ability of test agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
the Toxicity of Conditioning to Lower the Barrier for Achieving a HSC Transplant Therapy for HIV / AIDS
[0321]Hematopoietic stem cell (HSC) transplantation is the one known basis for apparent cure of HIV. The ‘Berlin patient’ in whom an allogeneic CCR5− / − hematopoietic graft after intensive chemotherapy provided a durable state of undetectable HIV, provides strong rationale for a stem cell based approach.1 Recently, two additional patients treated with allogeneic transplantation were reported to have undectable HIV viral load at 8 and 17 months post-transplant. (Abstract THAA0101 XIX International AIDS Conference, Washington, D.C.; Jul. 22-27, 2012) In addition, genetically modified HSC to enhance HIV resistance have been tested in clinical trials.2 (and unreported Systemix sponsored multi-center trial, DTS as investigator) Multiple efforts to leverage emerging gene modification strategies such as TALENs and zinc-finger nucleases are focused on creating HIV resistant autologous HSC tha...
example 2
iche Vacancy without Niche Toxicity Through Manipulation of CXCR2 and CXCR4
[0326]Preclinical data suggests that CXCR2 agonists may be superior to current standard of care both in terms of the kinetics of action and the quality of cells that get mobilized. The goal of this project is to test existing GSK CXCR2 agonists, Gro-beta in combination with the CXCR4 inhibitor Mozobil®, and compare outcomes to current standard of care for mobilization, engraftment, and as non-toxic conditioning regimens. Animals will be treated with various combinations of G-CSF, Plerixafor (e.g., Mozobil®), Gro-beta or other mobilizing agents and tested for their ability to induce HSC mobilization. Cells mobilized will be analyzed for their in vitro & in vivo functional capacities and ability to enhance survival and hematopoietic recovery in irradiated mice. Cells will be characterized via functional assays, immunohistochemistry and transcriptomics to help define variations in mobilized stem cell populations...
example 3
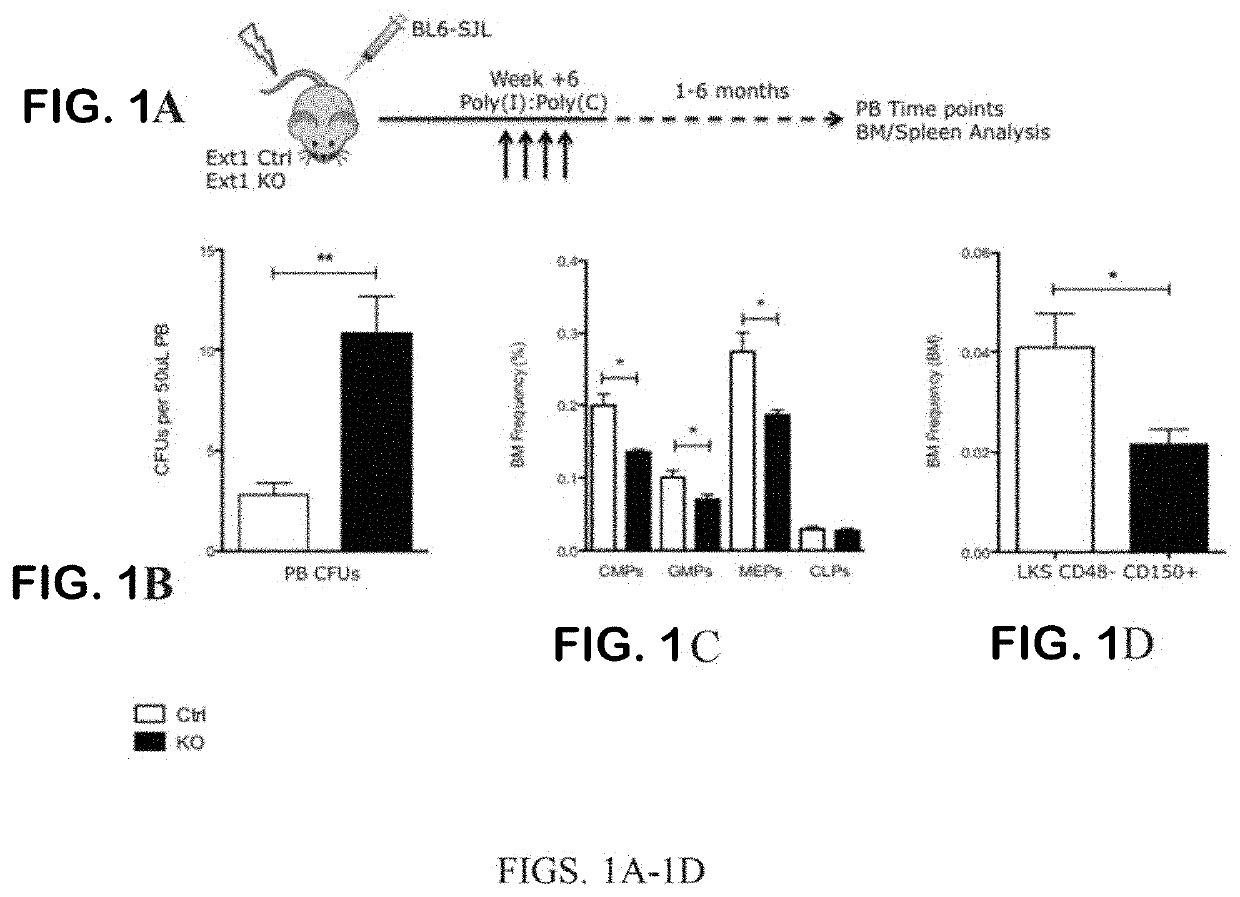
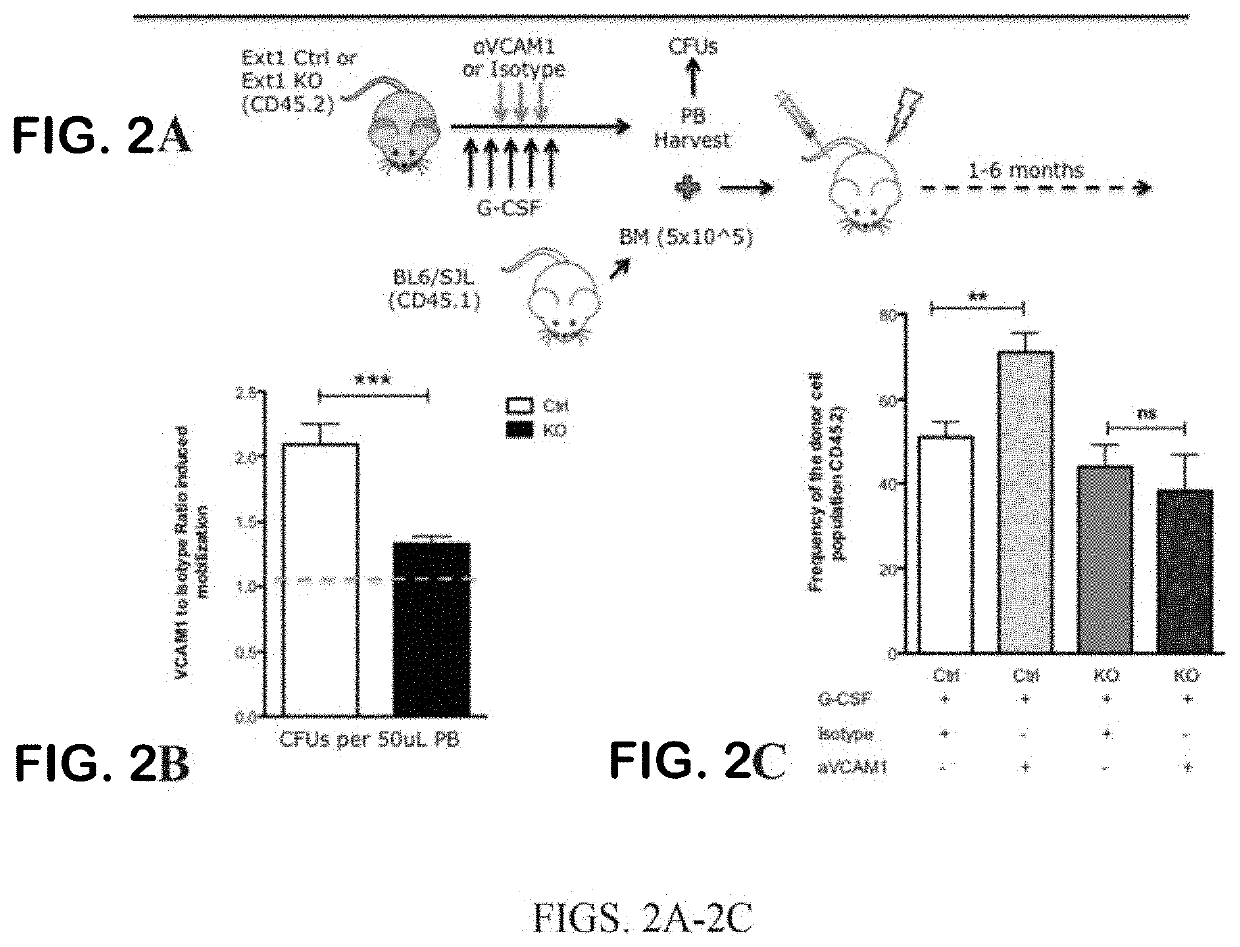
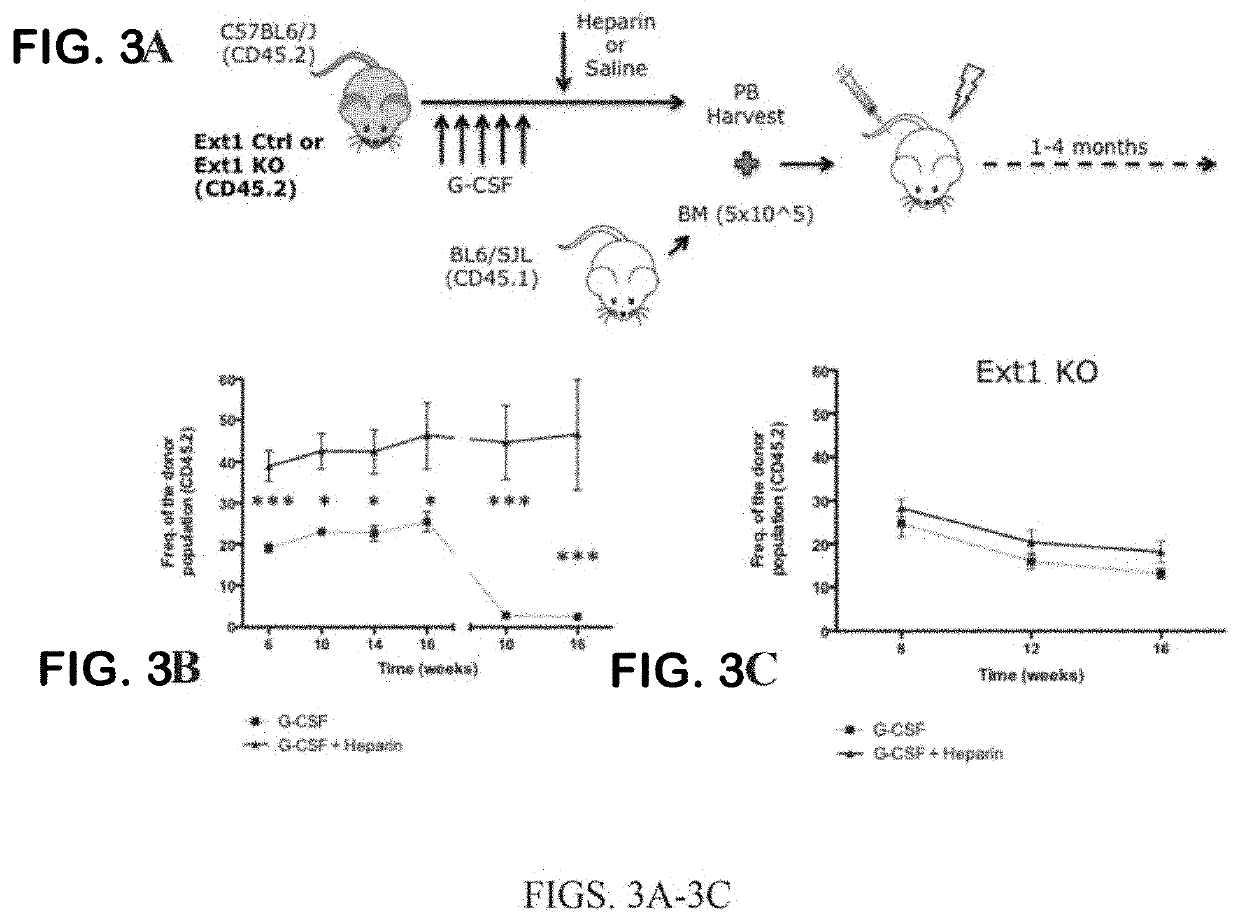
iche Vacancy without Niche Toxicity Through Manipulation of CXCR2 and Recently Defined Heparan Sulfate Proteoglycan Interactions Between HSC and the Bone Marrow Niche
[0333]Niche retention of HSPC is partially maintained by the interaction of SDF-1 with its cognate receptor CXCR4 on HSPC. Clinically, the CXCR4 antagonist AMD3100 is FDA approved for use in combination with G-CSF to enhance egress of HSPC from the bone marrow to the periphery for harvesting via apheresis and subsequent transplantation. While this combination clearly vacates the bone marrow niche, G-CSF causes significant attenuation of stromal niche cells.18 This is of little consequence when the goal is simply stem cell harvesting, but it is problematic when considering vacating the niche to enable competing cells to engraft in the setting of low toxicity conditioning. This may be why G-CSF has been unsuccessful in this context; it reduces the supportive capacity of the niche for infused and endogenous cells. Therefor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com