Method of Treating a Child with Central Precocious Puberty using an Extended Release Composition
a technology of precocious puberty and composition, which is applied in the direction of endocrine system disorder, pharmaceutical non-active ingredients, infusion syringes, etc., can solve the problems of inconvenient effective period, inconvenient preparation and administration, and insufficient/inconvenient treatment period, so as to reduce the mean bone age of pediatric patients, reduce the mean bone growth velocity, and reduce the mean bone age of children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
rocedure for Preparation of Biodegradable Polymer
[0133]In a jacketed stainless steel polymerization vessel, appropriate amounts of lactide and glycolide are added and the vessel contents are placed under a nitrogen atmosphere. The temperature of the vessel is increased until the reagents melt. An appropriate amount of an alkanediol is then added, followed by addition of stannous octanoate catalyst. The vessel is then heated at about 135-145° C. under nitrogen atmosphere for about 3-4 hours with constant stirring. Then, to remove unreacted lactide and glycolide monomers, the vessel is evacuated and the monomers are vacuum distilled out of the polymerization mixture. The hot melt is then extruded into cooling pans. After cooling, the solid mass is cryo-ground to a fine powder and dried.
example 2
on and Administration of a Leuprolide Acetate Extended Release Composition
[0134]An extended release composition for use as a subcutaneous in situ depot in a pediatric patient 2 years of age or older for use as an effective treatment for CPP was prepared using a biodegradable polymer as prepared in Example 1. Components of the extended release composition of the invention are detailed in Table 1 below:
TABLE 1Extended Release Composition Reconstituted Drug ProductReconstitutedLeuprolide acetate delivered 45 mgDrug ProductApproximate leuprolide free base 42 mgequivalentPLG polymer delivered165 mgNMP delivered165 mgApproximate administered375 mgformulation weightApproximate injection volume0.375 mL
[0135]The extended release composition is comprised of 165 mg of 85:15 poly(D,L-lactide-co-glycolide) (PLG) copolymer segments dissolved in 165 mg of NMP organic solvent. The biodegradable polymer is polymerized using a 1,6-hexanediol core unit, and is therefore hydroxyl terminated at its dist...
example 3
h Clinical Study for Treating Children with CPP
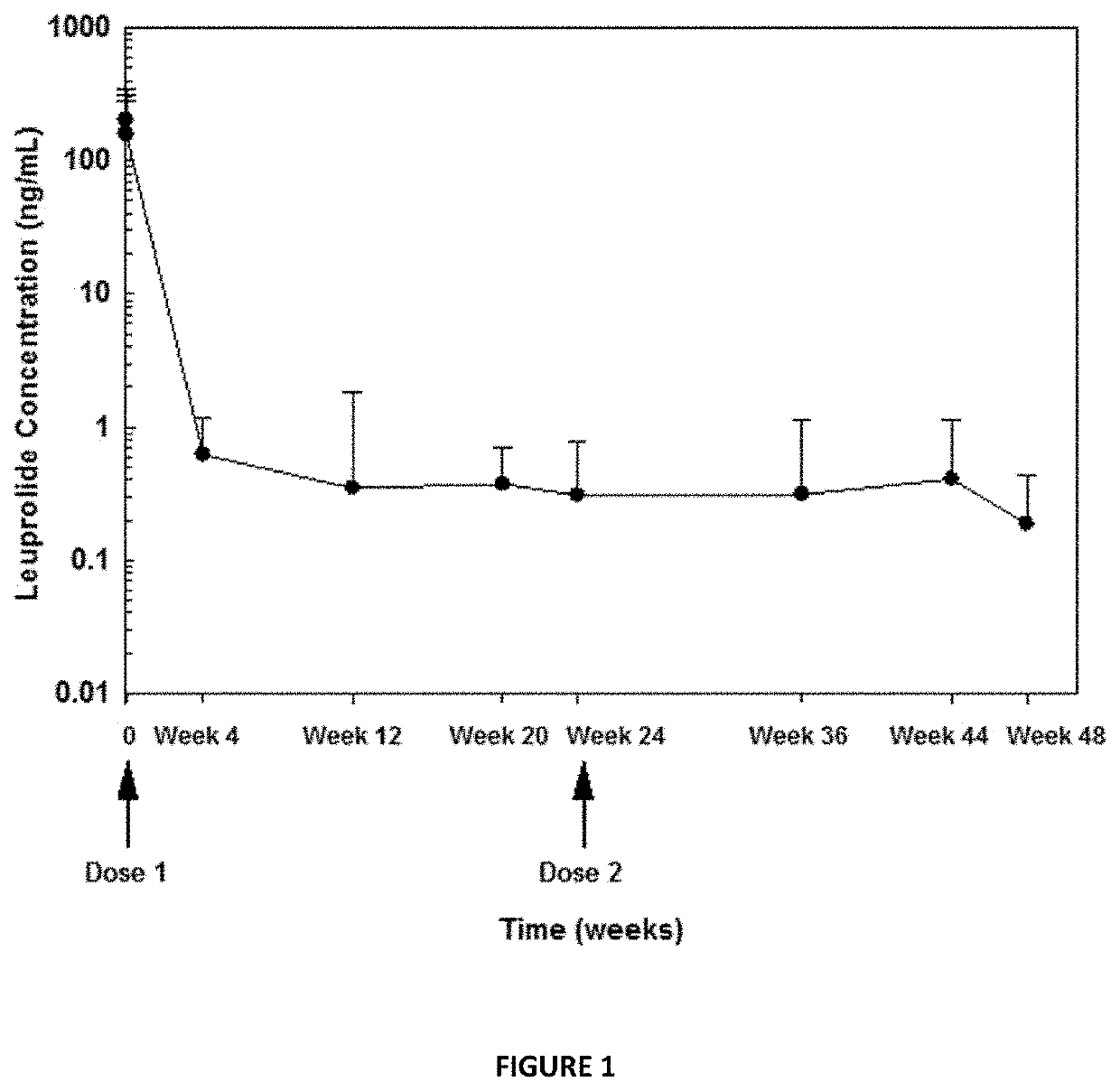
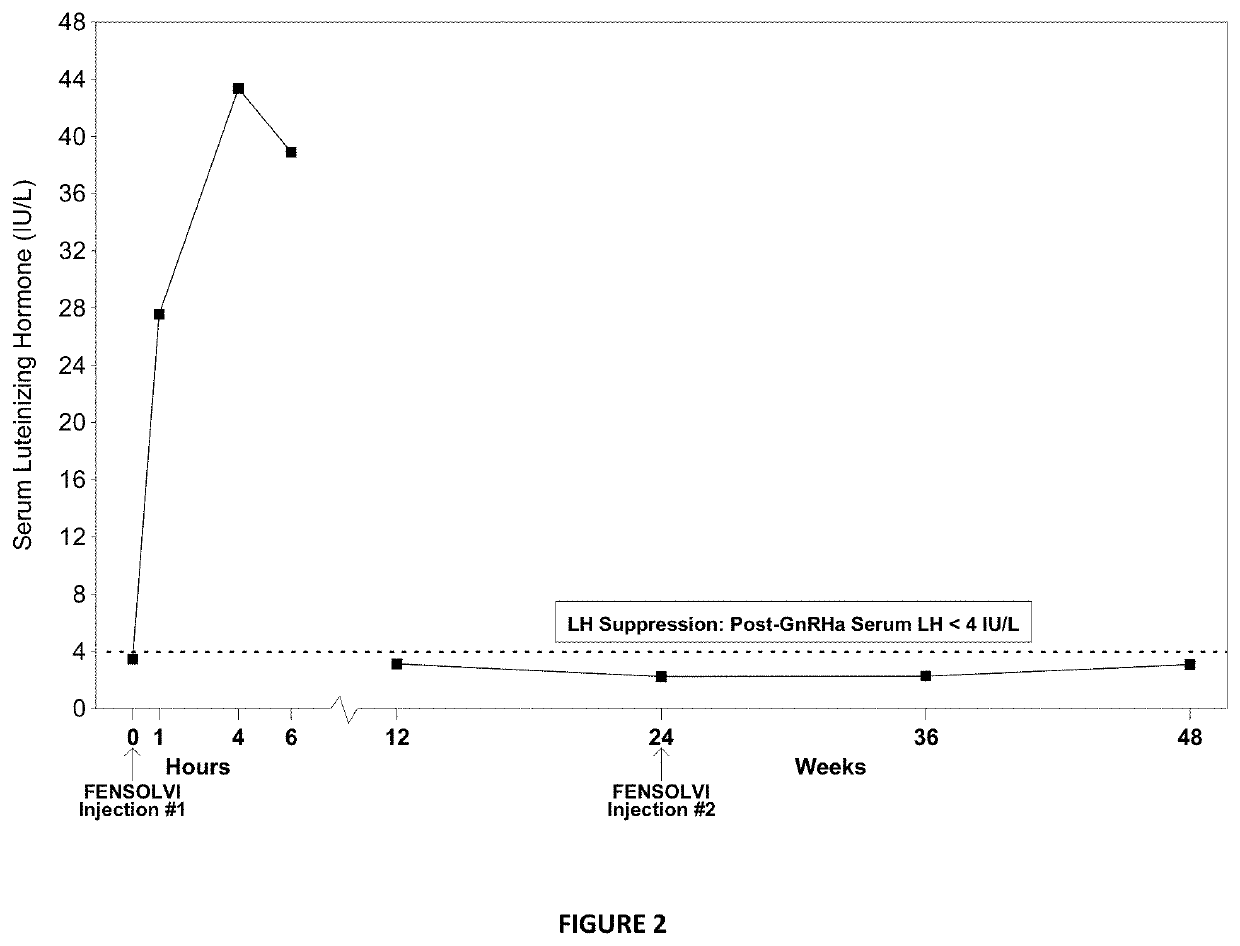
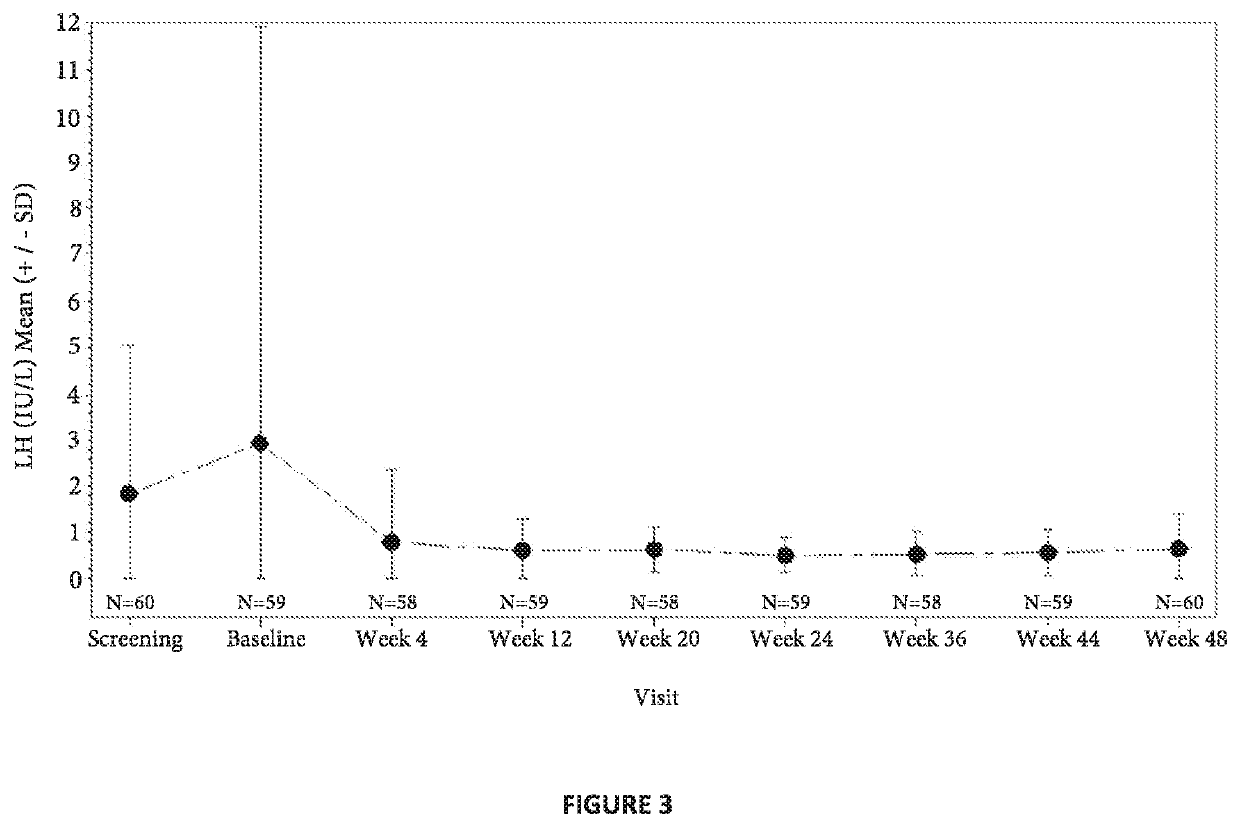
[0137]A multi-center, open-label, single arm, adaptive design was conducted to evaluate the efficacy and safety of the extended release composition described in Example 2 in treating pediatric patients 2 years of age or older with CPP. 114 male and female children suspected of having CPP, but who had not yet ever been treated with GnRH agonist based therapies, were administered a subcutaneous injection of a stimulation composition consisting of leuprolide acetate solution at a dose of either 20 μg / kg or 500 μg total depending on physician discretion, in order to confirm an initial diagnosis of CPP. Of the 114 subjects enrolled and screened, 50 children did not receive any dose of the extended release composition as they failed to meet additional screening criteria beyond confirmed CPP diagnosis and where therefore excluded from the instant clinical trial. The mean age was 7.5 years (range 4-9 years) at the start of treatment. Additional...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap