Delivery of payloads to stem cells
a stem cell and payload technology, applied in the direction of genital tract cells, skeletal/connective tissue cells, peptide/protein ingredients, etc., can solve the problems of difficult patient eating, severe side effects, and difficult for patients, especially children, to cope with
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
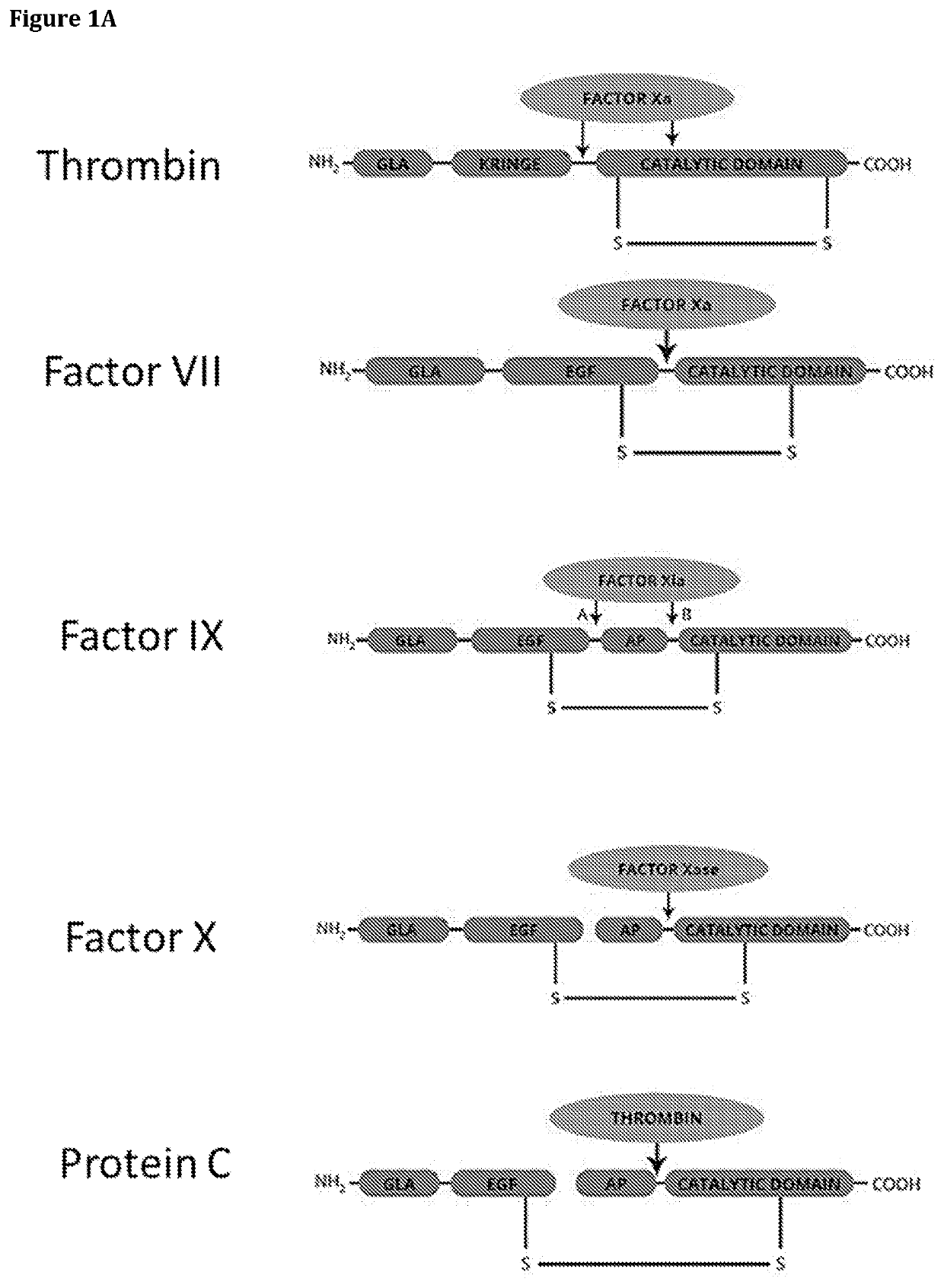
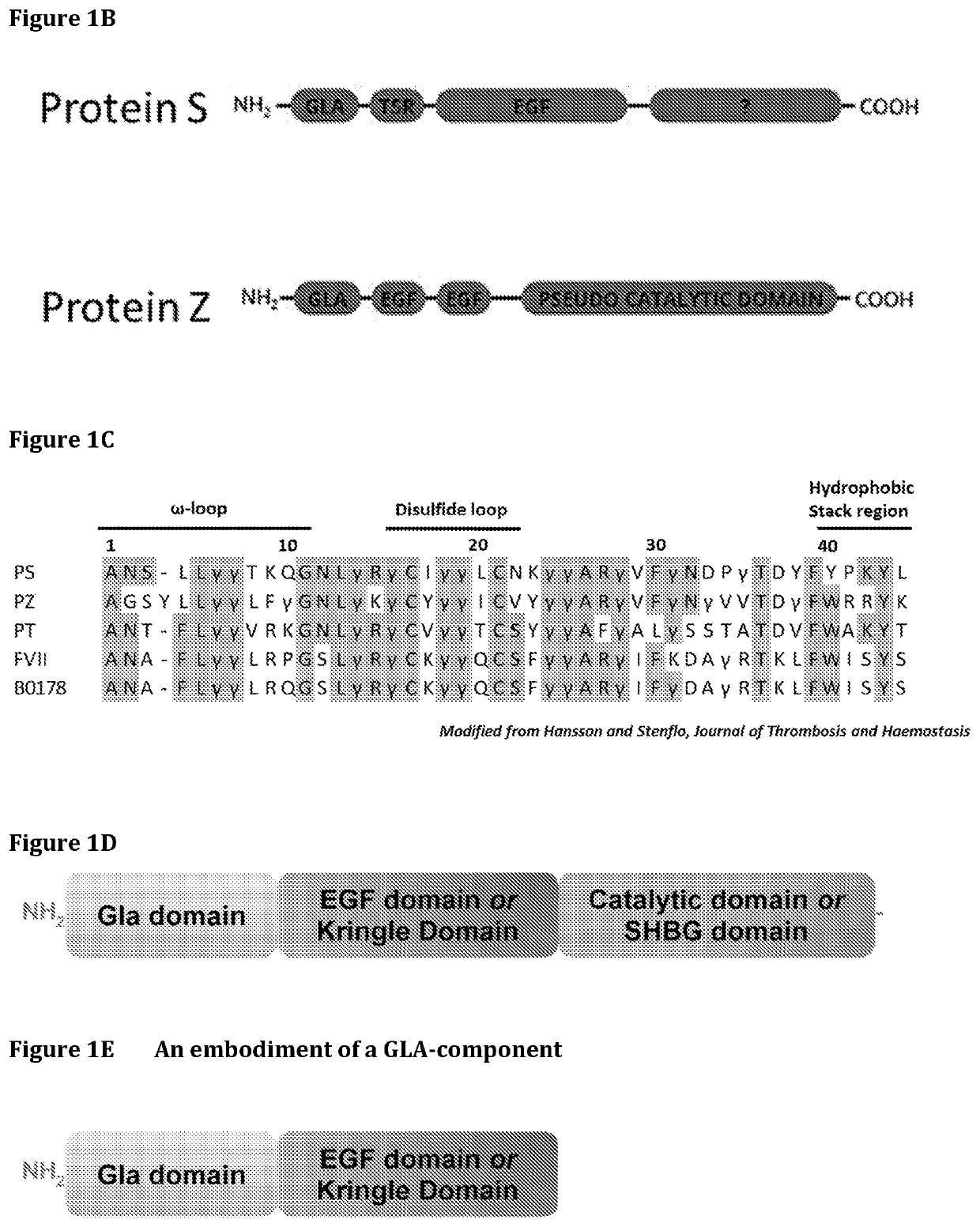
[0245]FIG. 1A-D Shows various representations of GLA protein structures.
[0246]FIG. 1E Shows an embodiment of a GLA-component according to the present disclosure.
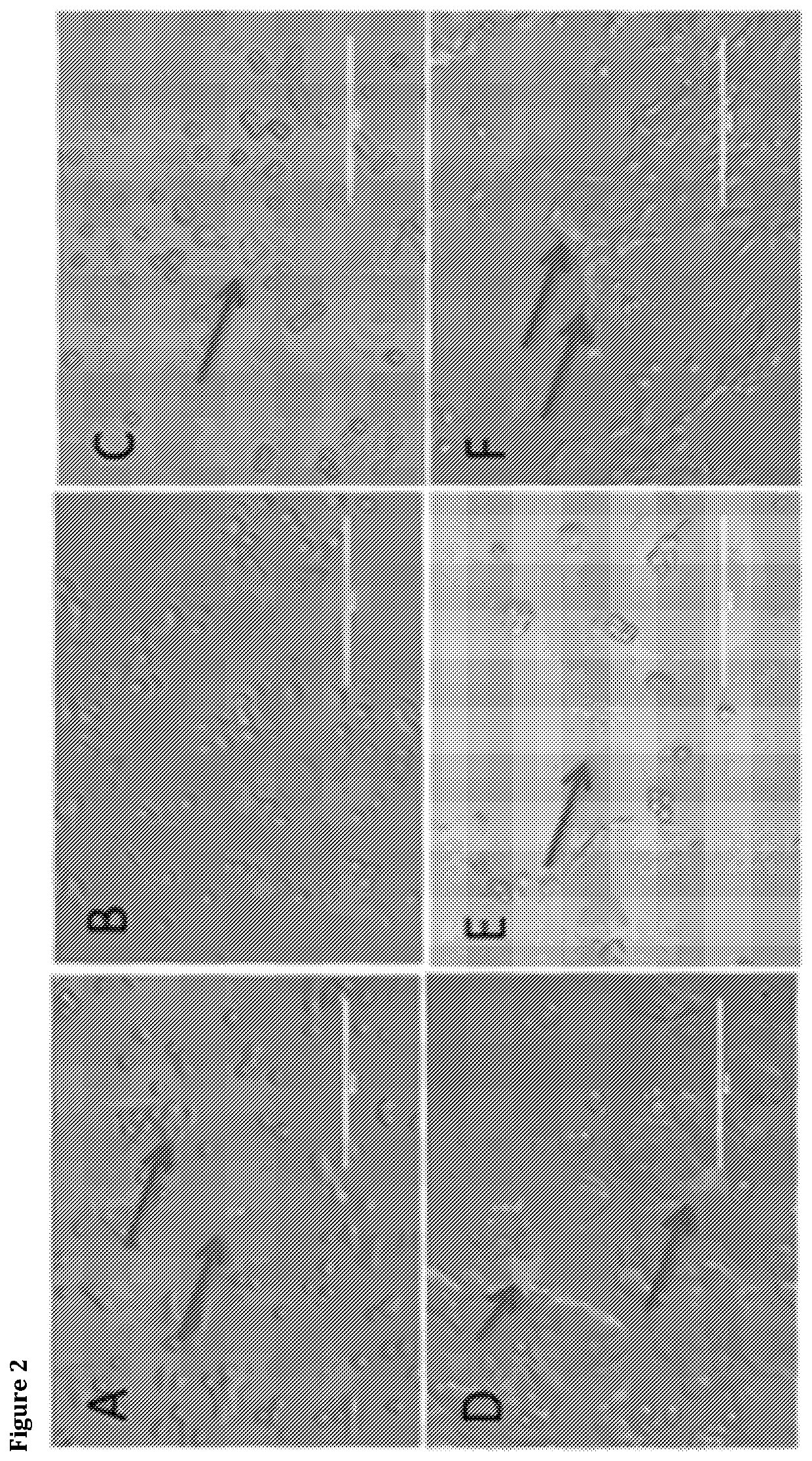
[0247]FIG. 2 Shows Protein S (PrS) and annexin staining of breast cancer cell lines treated with peroxide to induce apoptosis. A, human MDA-231 cells treated with peroxide and stained with FITC-PrS. B, untreated MDA-231 cells stained as in A. C, treated MDA-231 cells stained with annexin. D, human MCF-7 cells treated with peroxide and stained with PrS. E, murine MET-1 cells, as in D. F, murine 4T1 cells, as in D.
[0248]FIG. 3 Shows overlapping, yet distinct, cellular localization of PrS and annexin. A, murine 4T1 cells treated with peroxide and stained with Cy5 PrS (RED) and FITC annexin (GREEN). Light arrow, co-localized signals; red arrows, cells staining with PrS and not annexin; green arrow, cell staining relatively brighter with annexin but less bright with PrS, indicating distinct binding patterns (insets show PrS and a...
example 2
[0290]Stem cells are distinct in phenotype from differentiated cells and may express PS non-apoptotically to avoid the induction of immune responses. Stem cells were stained with a GLA domain molecule of the present disclosure comprising a payload of a fluorescent label, without the induction of apoptosis.
[0291]Trophoblast stem cells, (FIG. 14) which differentiate into several types of trophoblasts in the placenta, stained with Protein S, whereas differentiated trophoblasts derived from these cells in culture did not stain. The stain was able to distinguish between in vivo differentiated stems cells and cells differentiated in vitro.
[0292]This data the molecules of the present disclosure may be employed to target cells in vivo or in ex vivo samples.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| surface | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com