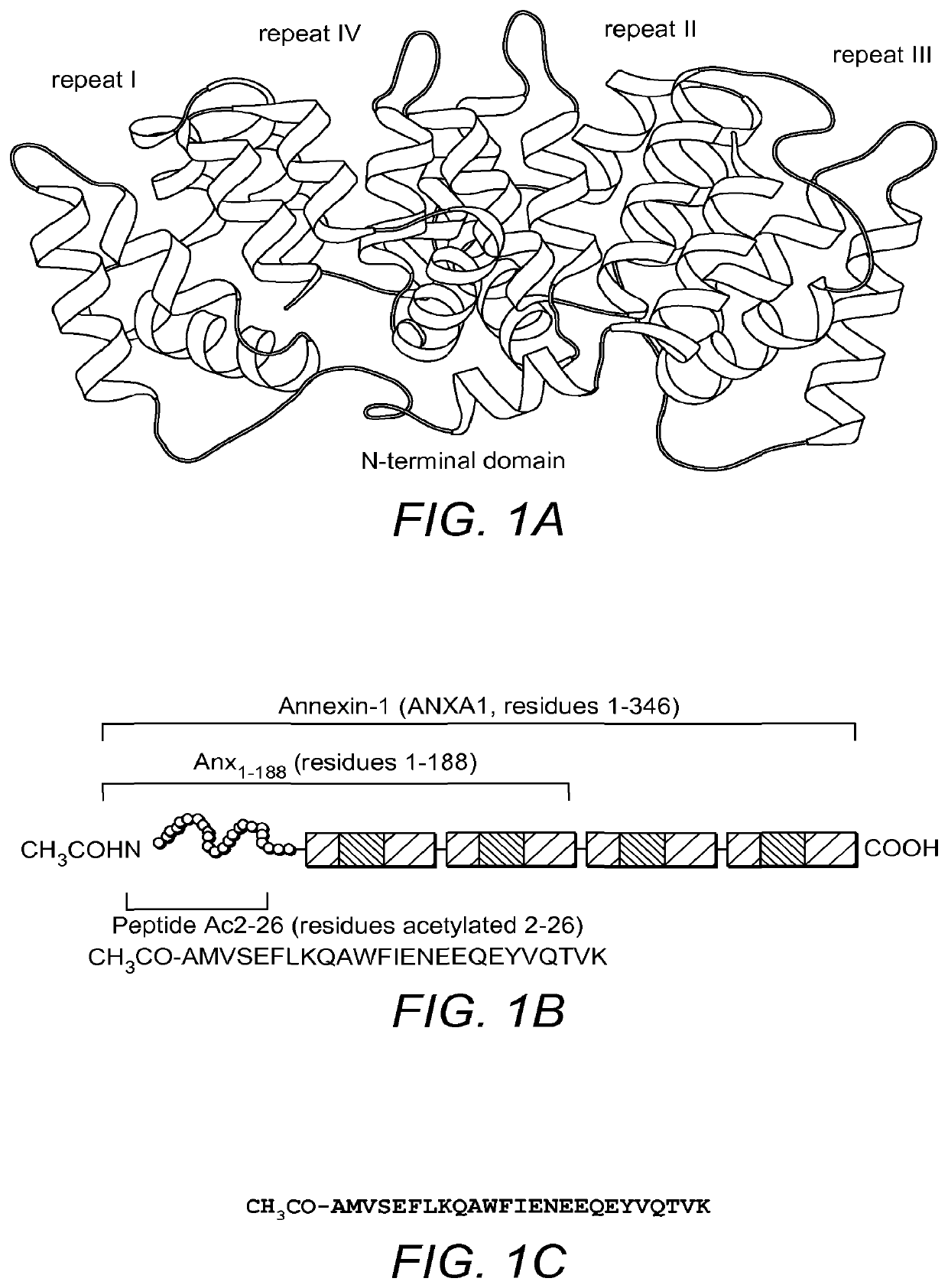
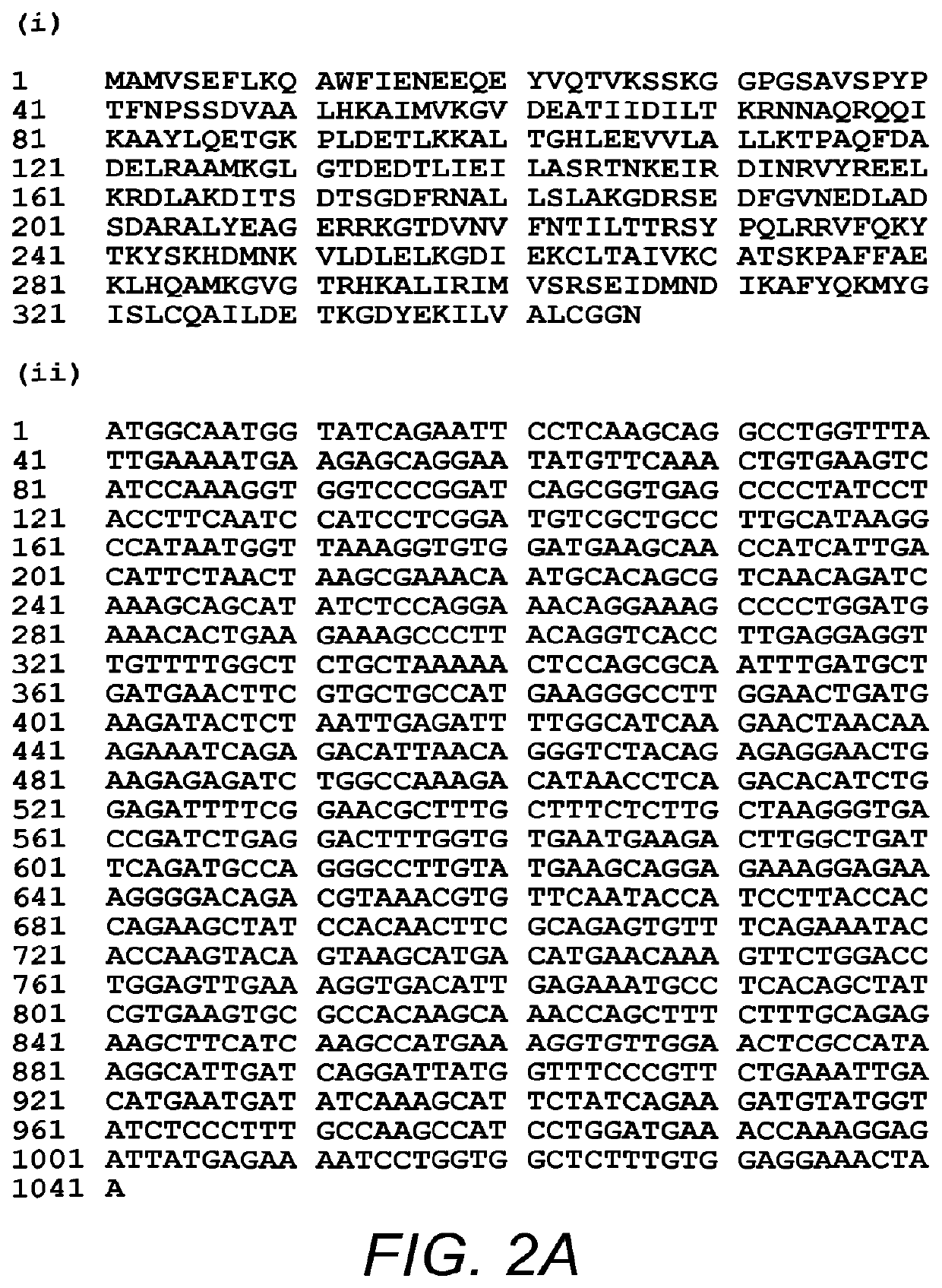
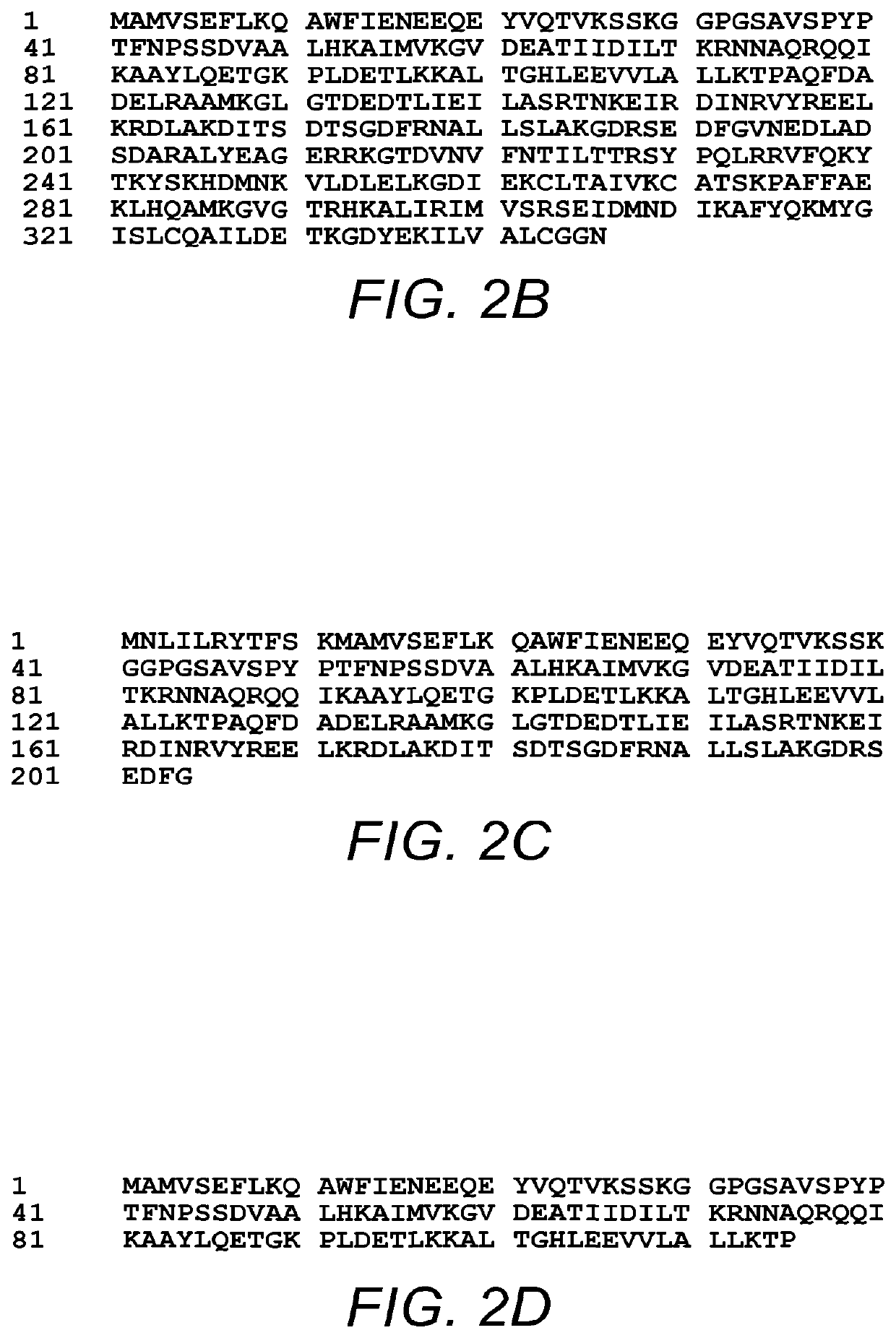
Use of Antibody
a technology of specific binding molecules and antibodies, applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve the problems of limiting the use of these drugs, and 30% of patients are refractory to pharmacotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N OF ANTIBODY VJ-4B6
[0102]A novel anti-AnxA1 antibody was generated by genetic immunisation as indicated in the scheme in FIG. 3A (Genovac GmbH, Germany). Serum from several immunized mice were tested and three resulted positive for IgG recognizing cells transfected with AnxA1 cDNA. Splenocytes from these mice were fused to myeloma cells to generate hybridoma cells. Only one of the three hybridoma cell clones were successfully subcloned and expanded. These hybridoma cells are called VJ-4B6-E5-B10-D4. Purified IgG2b fraction from the hybridoma cells recognizes cells transfected with AnxA1 cDNA (FIG. 3B, green line; right-hand peak) but not cell transfected with an irrelevant cDNA (FIG. 3B, red line; left-hand peak).
example 2
G OF VJ-4B6
[0103]The aim of this Example was to clone the antibody heavy and light chain variable region genes from the hybridoma cells and to determine the DNA sequence and location of the complementarity determining regions (CDRs) and other features.
[0104]Cloning and Sequencing of Antibody Variable Regions
[0105]Total RNA was prepared from 1 vial of hybridoma cells using the Qiagen RNeasy mini kit (Cat No: 74104). RNA was eluted in 50 μL water and checked on a 1.2% agarose gel.
[0106]VH and VK (variable kappa light chain) cDNAs were prepared using reverse transcriptase with IgG and kappa constant region primers. The first strand cDNAs were amplified by PCR using a large set of signal sequence primers. The amplified DNAs were gel-purified and cloned into the vector pGem® T Easy (Promega). The VH and VK clones obtained were screened for inserts of the expected size. The DNA sequence of selected clones was determined in both directions by automated DNA sequencing. The locations of the ...
example 3
F VJ-4B6 ON MOUSE MODEL OF OBSESSIVE COMPULSIVE DISORDER (OCD) IN VIVO
[0114]The present inventors have developed a transgenic mouse model of obsessive compulsive disorder in which Anx-A1 is overexpressed in T cells. This is the subject of co-pending UK patent application no. 1121561.3 which was filed on the same day as the present application.
[0115]Transgenic mice overexpressing Anx-A1 (Anx-A1tg) in T cells were generated by inserting C-terminus FLAG-tagged Anx-A1 into the mouse genome using a VACD2 cassette vector and the technique of pronuclear microinjection. Briefly, the murine Anx-A1 gene was amplified and tagged with the FLAG epitope and then cloned into the pcDNA3.1 vector. The Anx-A1 FLAG was recovered from the pcDNA3.1 vector and then ligated into linearised VACD2 vector. The VACD2 Anx-A1 FLAG construct was then modified and purified, then inserted into the mouse genome by pronuclear injection. These mice were used in the present Example as follows.
[0116]Mice received an in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com