Derivatives of 10-methylene lipids, process for preparing such derivatives and use thereof
a technology of methylene lipids and derivatives, which is applied in the preparation of carboxylic compounds, biocide, organic chemistry, etc., can solve the problems of low production yield related to total lipid content, preventing further industrial use, and unable to achieve a wide range of purification methods suitable for industrial scale production at reasonable cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
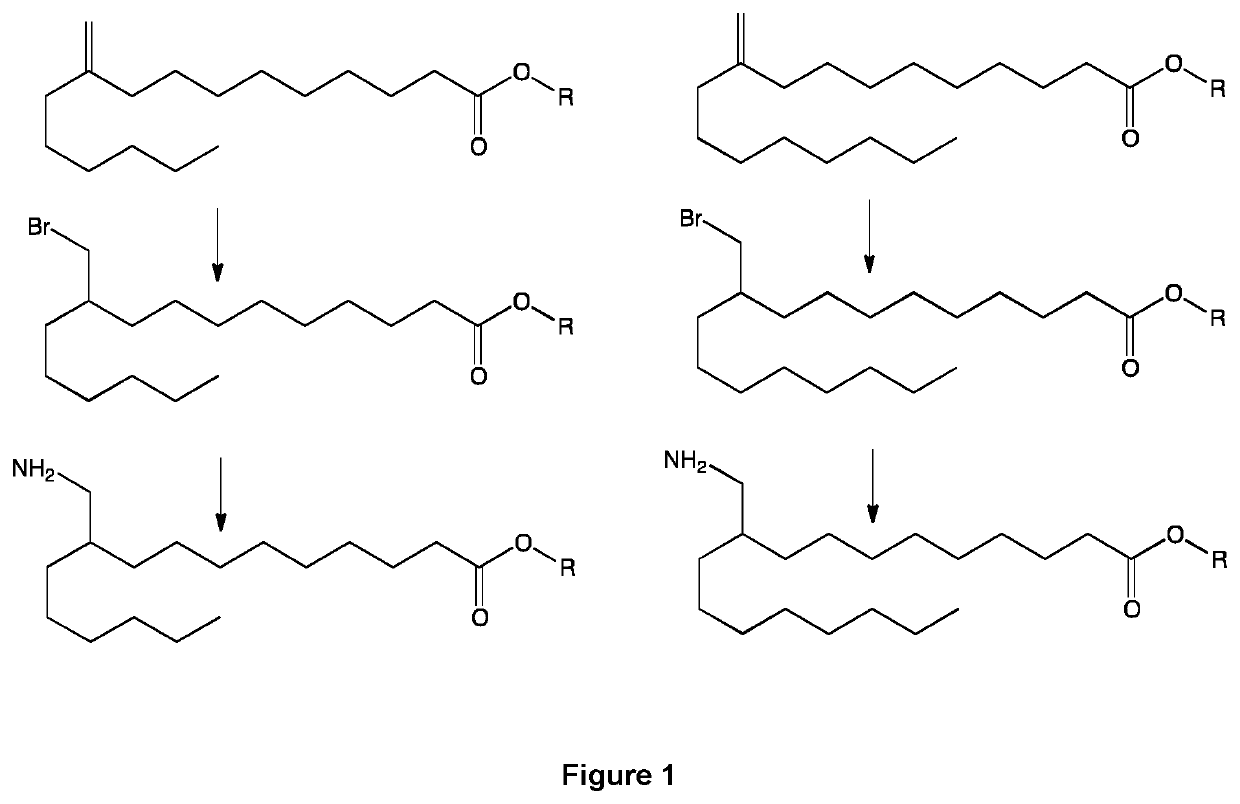
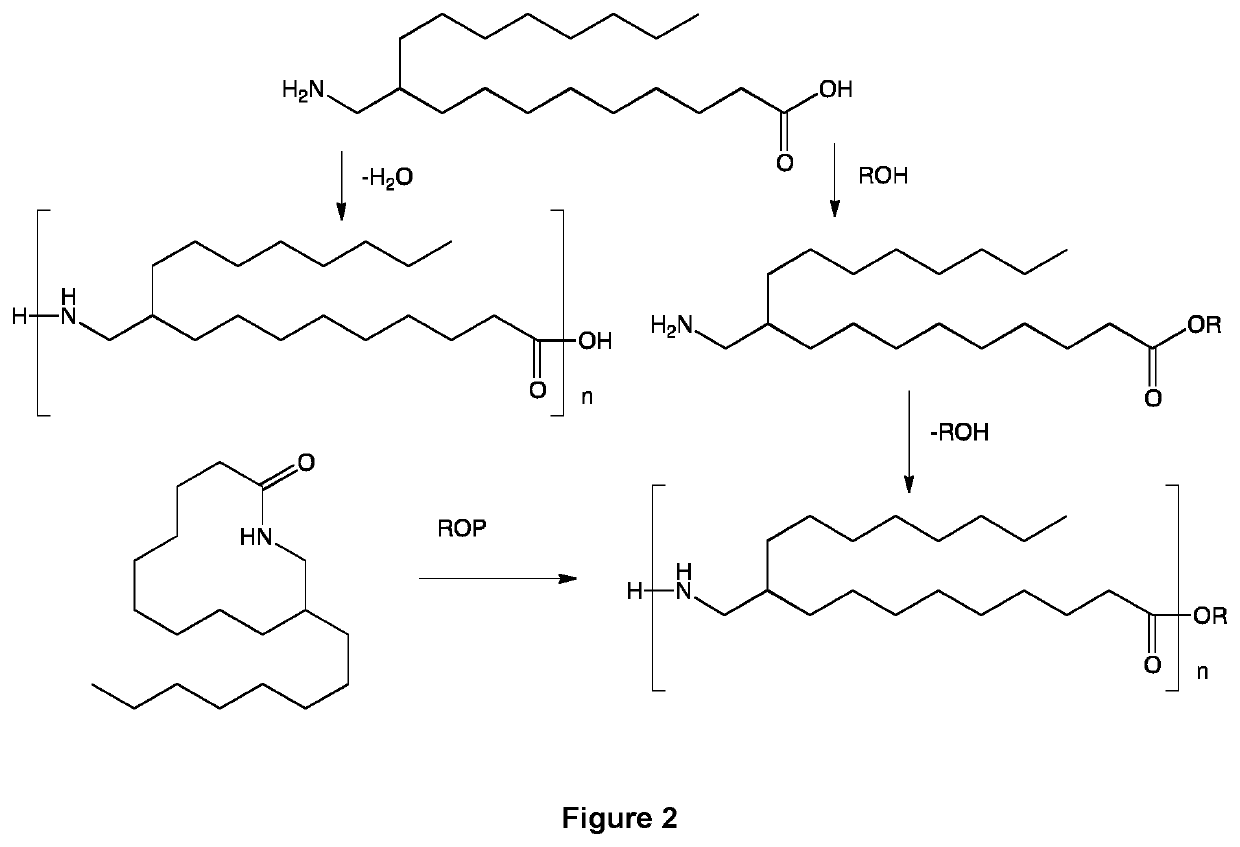
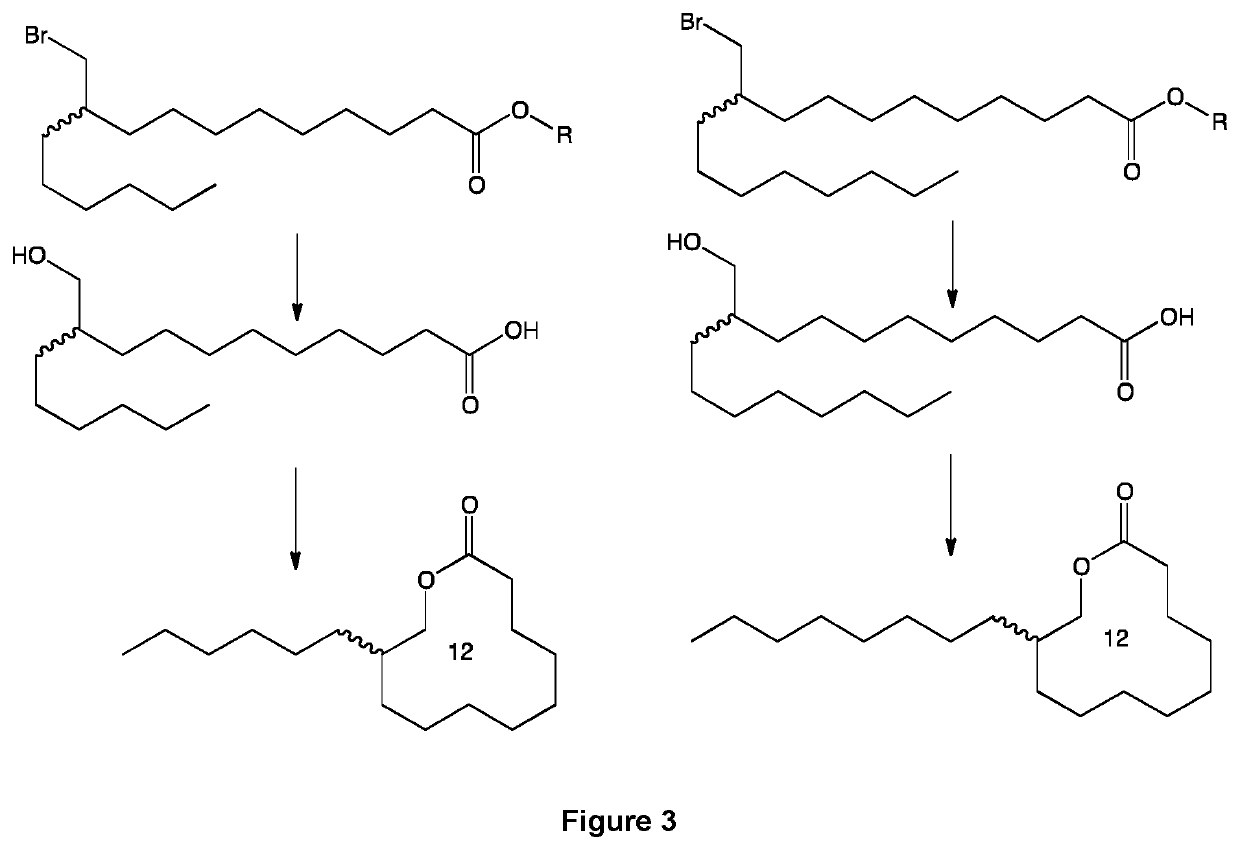
[0087]The embodiments of the present invention will be better understood by looking at the different examples below, illustrated by the following figures showing preferred pathways and products preparation schemes and methods. On all the figures, R represents an alkyl group, for example chosen from a methyl, ethyl, propyl and isopropyl group.
[0088]FIG. 1 represents a pathway from 10-methylene-palmitic ester / acid to 10-bromomethyl-palmitic ester / acid and 10-aminomethyl-palmitic ester / acid (left side), and 10-methylene-stearic ester / acid to 10-bromomethyl-stearic ester / acid and 10-aminomethyl-stearic ester / acid (right side). R may represent an alkyl or hydrogen group.
[0089]FIG. 2 represents the polycondensation of 10-aminomethyl-stearic acid to a polyamide, directly with water elimination or via the formation of the corresponding 10-aminomethyl-stearic ester. In the figure, n represents the number of units of the polyamide. Also shown on bottom left is ring opening polymerization (ROP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com