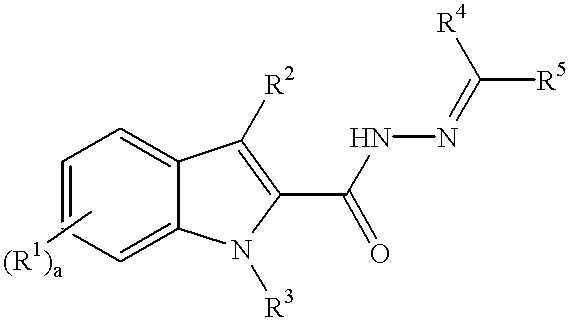
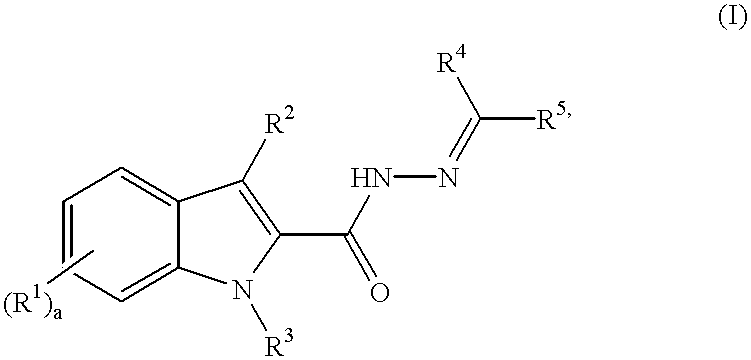
3-substituted indole angiogenesis inhibitors
a technology of indole carbohydrazide and indole carbohydrazide, which is applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problem of not being developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N'-((4-methoxyphenyl)methylidene)-3-phenyl-1H-indole-2-carbohydrazide
example 1a
ethyl 2-oxo-3-phenylpropanoate
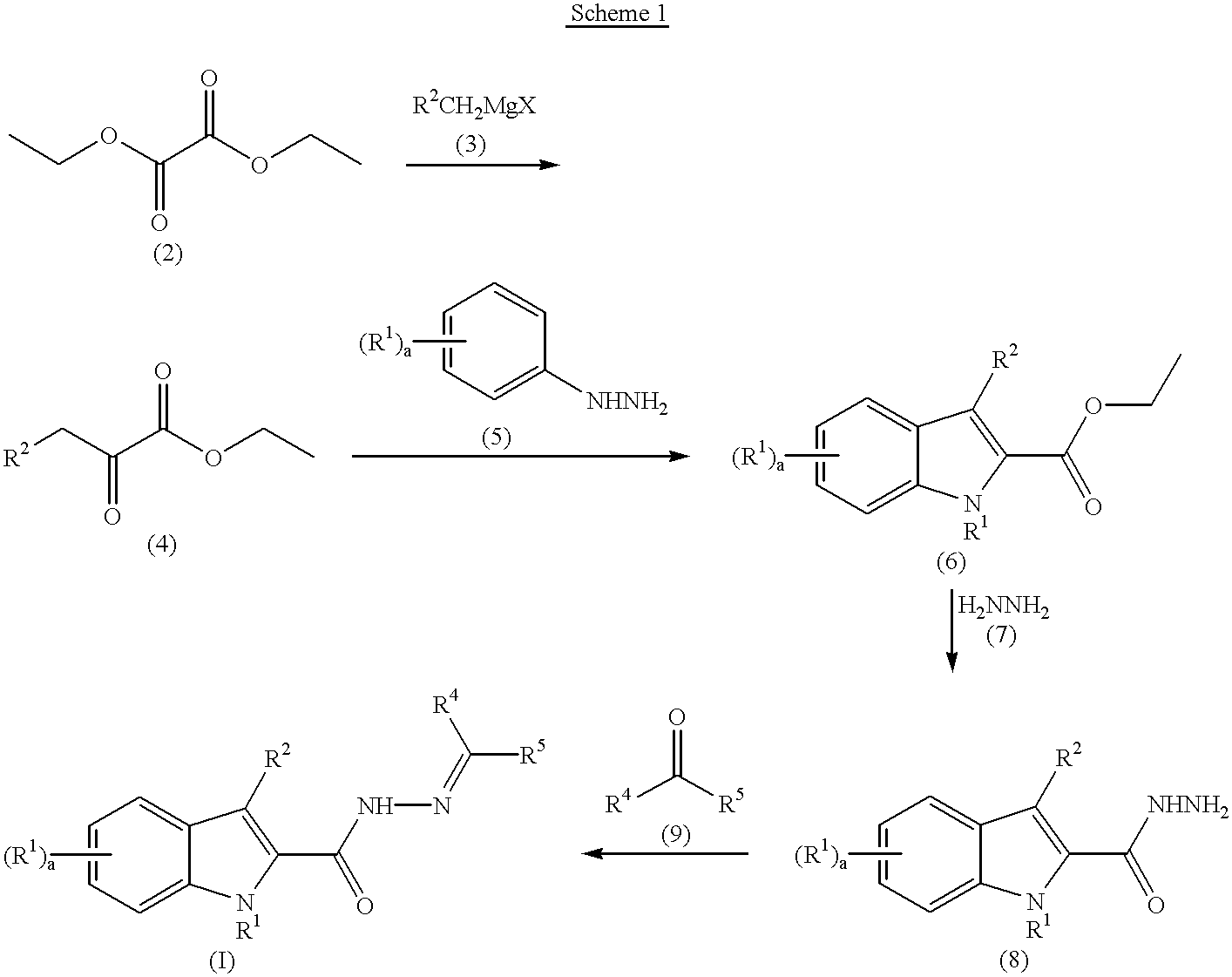
A solution of diethyl oxalate (11.15 mL, 82.1 mmol) in diethyl ether (50 mL) at -78.degree. C. was treated dropwise with 1 M benzylmagnesium chloride in diethyl ether (82 mL, 82 mmol) while maintaining an internal temperature of -60.degree. C. The mixture was stirred for 30 minutes and poured into a mixture of concentrated HCl (8 mL), ice (40 mL), and diethyl ether (50 mL). The organic phase was washed with water and brine, dried (MgSO.sub.4), filtered, and concentrated to provide 15.5 g (98%) of the desired product of sufficient purity for subsequent use.
example 1b
ethyl 3-phenyl-1H-indole-2-carboxylate
A mixture of Example 1A (7.81 g, 40.7 mmol) and phenylhydrazine (4.00 mL, 40.7 mmol) was treated with concentrated sulfuric acid (4 drops), heated to 120.degree. C. for 15 minutes, cooled to room temperature, treated with ethanol (50 mL), treated with bubbling HCl gas for 2 minutes, and heated to reflux for 1 hour. The mixture was poured into water (100 mL) and extracted with diethyl ether. The combined extracts were washed with water and brine, dried (Na.sub.2 SO.sub.4), filtered, and concentrated. The concentrate was recrystallized from ethanol to provide 3.43 g (32%) of the desired product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com