Receptor tyrosine kinase signaling pathway analysis for diagnosis and therapy
a receptor tyrosine kinase and signaling pathway technology, applied in the field of receptor tyrosine kinase signaling pathway analysis for diagnosis and therapy, can solve the problems of lack of sensitivity, difficult application of techniques, and difficulty in studying the signaling pathway, and achieve the effect of overcompensating deficiencies in the ar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Simultaneous Measurement of Her2-Her3 Heterodimerization and Erk1 Phosphorylation
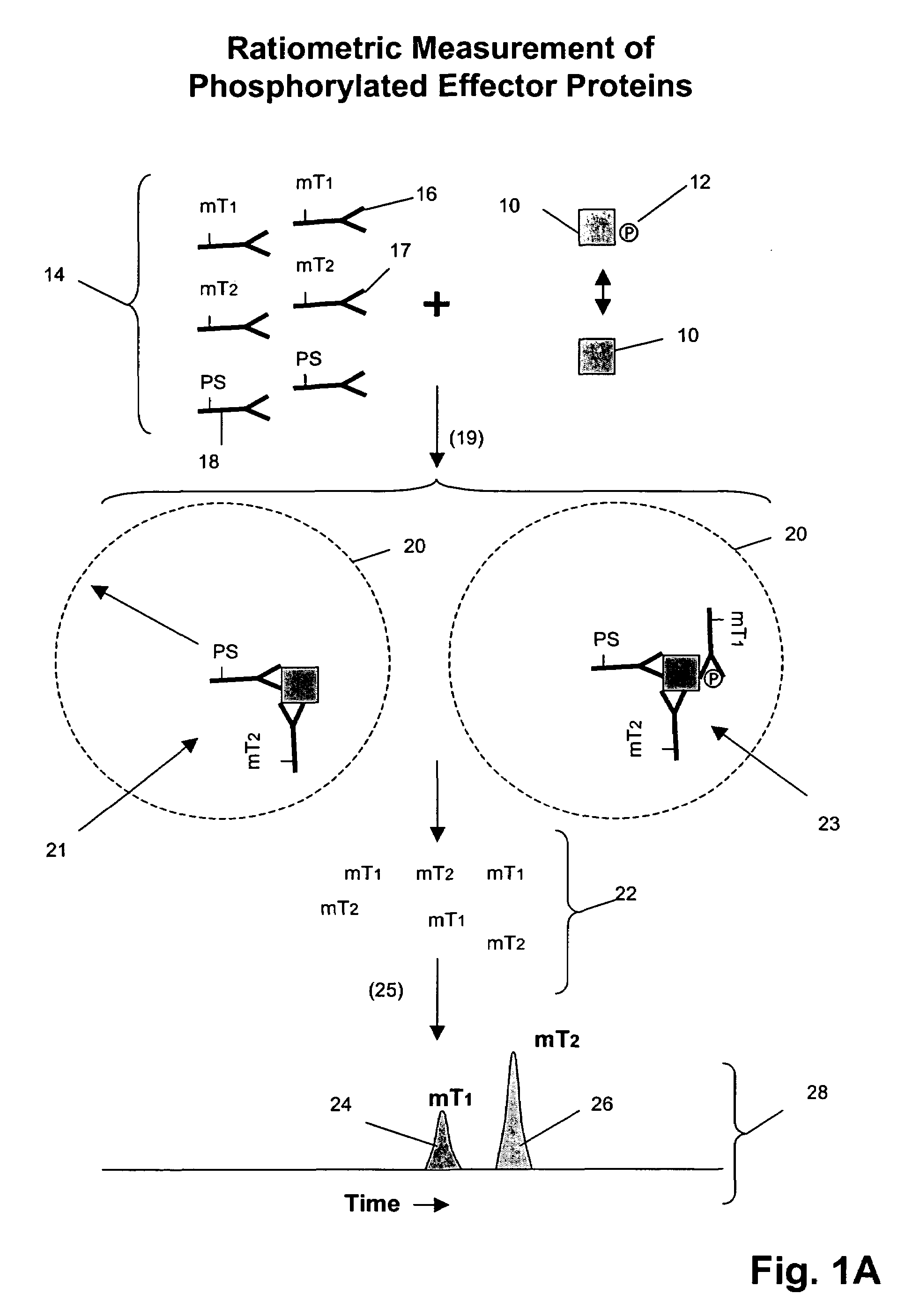
[0143]In this example, an assay is described for providing a ratiometric measure of phosphorylated Erk1 and Her2-Her3 heterodimerization. The assays are carried out as follows.
[0144]1. Serum-starve breast cancer cell line culture (MCF-7) overnight before use.[0145]2. Stimulate cell lines with HRG in culture media for 10 minutes at 37° C. Exemplary doses of HRG are 0, 0.032, 0.16, 0.8, 4, 20, 100 nM for MCF-7 cells.[0146]3. Aspirate culture media, transfer onto ice, and add lysis buffer (described below) to lyse cells in situ.[0147]4. Scrape and transfer lysate to microfuge tube. Incubate on ice for 30 min. Microfuge at 14,000 rpm, 4° C., for 10 min.[0148]5. Collect supernatants as lysates and aliquot for storage at −80° C. until use.
Lysis Buffer (Made Fresh and Stored on Ice):
[0149]
FinalulStock1% Triton X-100100010% 20 mM Tris-HCl (pH 7.5) 200 1 M100 mM NaCl 200 5 M 50 mM NaF 500 1...
example 2
Analysis of Cell Lysates for Her-2 Heterodimerization and Receptor Phosphorylation
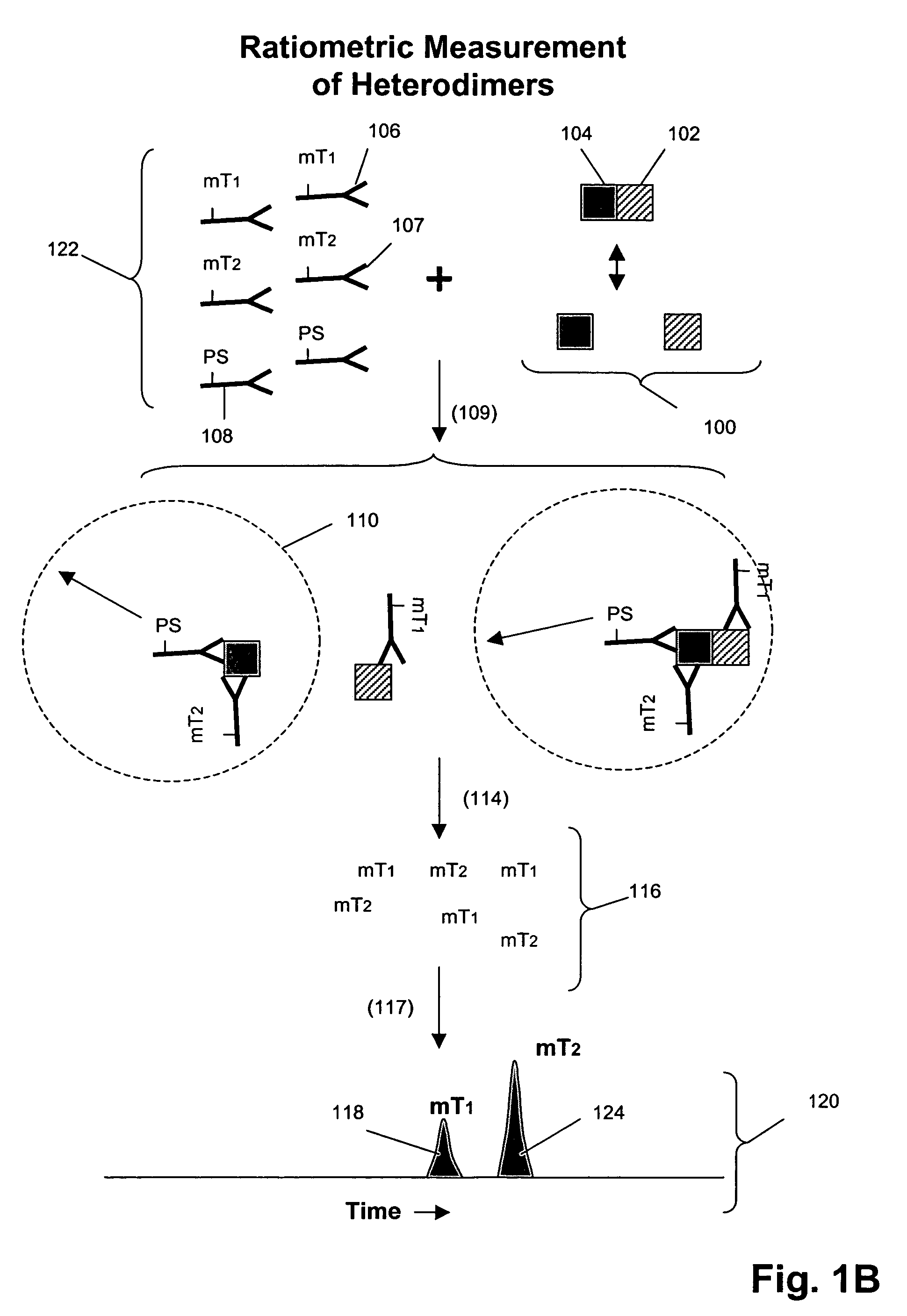
[0167]In this example, Her1-Her2 and Her2-Her3 heterodimers and phosphorylation states are measured in cell lysates from several cell lines after treatment with various concentrations of epidermal growth factor (EGF) and heregulin (HRG). Measurements are made using three binding compounds and a cleaving probe as described below.
[0168]1. Serum-starve breast cancer cell line culture overnight before use.[0169]2. Stimulate cell lines with EGF and / or HRG in culture media for 10 minutes at 37° C. Exemplary doses of EGF / HRG are 0, 0.032, 0.16, 0.8, 4, 20, 100 nM for all cell lines (e.g. MCF-7, T47D, SKBR-3) except BT20 for which the maximal dose is increased to 500 nM because saturation is not achieved with 100 nM EGF.[0170]3. Aspirate culture media, transfer onto ice, and add lysis buffer to lyse cells in situ.[0171]4. Scrape and transfer lysate to microfuge tube. Incubate on ice for 30 m...
example 3
Analysis of Tissue Lysates for Her2 Heterodimerization and Receptor Phosphorylation
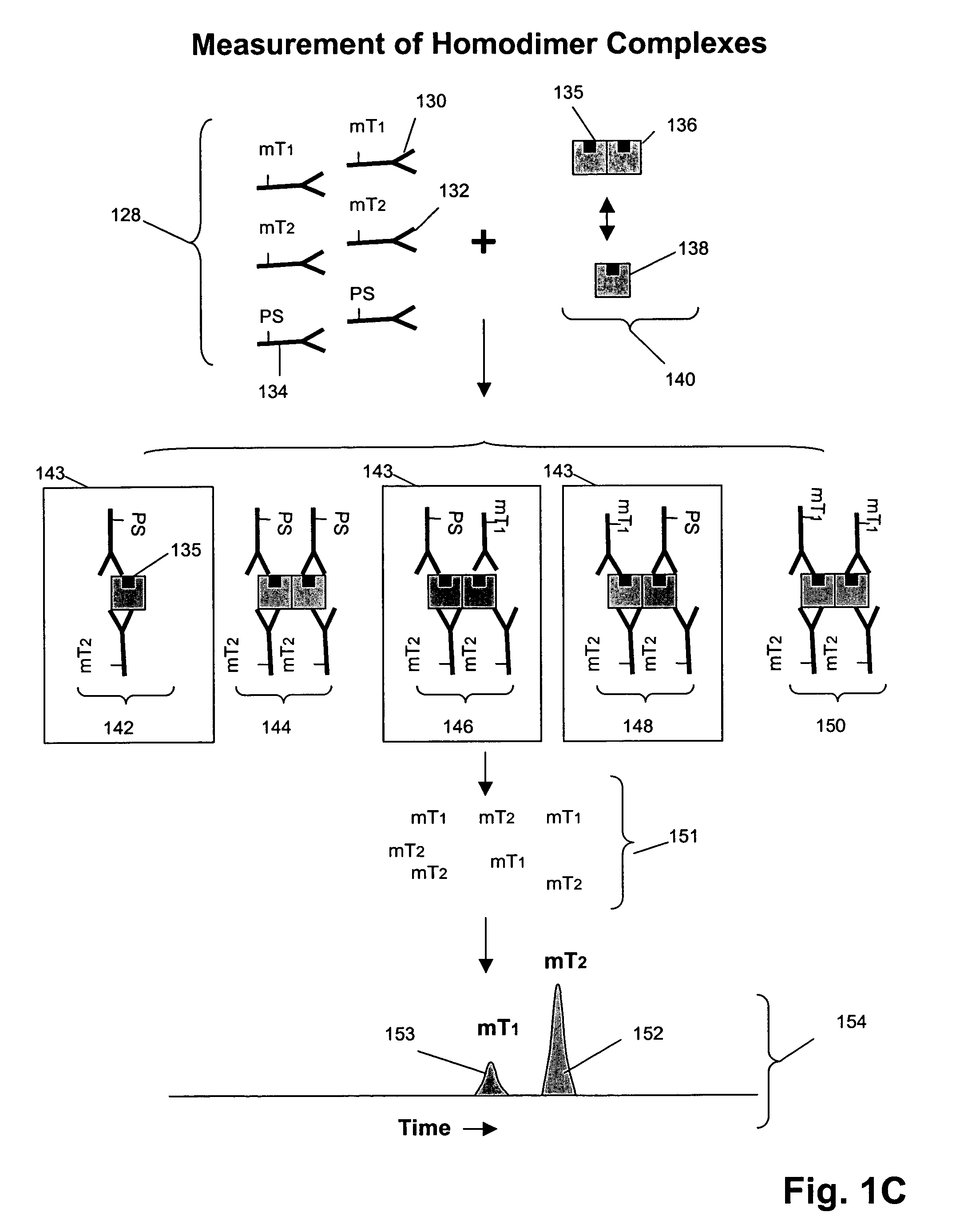
[0193]In this example, Her1-Her2 and Her2-Her3 heterodimers and phosphorylation states are measured in tissue lysates from human breast cancer specimens.
[0194]1. Snap frozen tissues are mechanically disrupted at the frozen state by cutting.[0195]2. Transfer tissues to microfuge tube and add 3×tissue volumes of lysis buffer (from appendix I) followed by vortexing to disperse tissues in buffer.[0196]3. Incubate on ice for 30 min with intermittent vortexing to mix.[0197]4. Centrifuge at 14,000 rpm, 4° C., for 20 min.[0198]5. Collect supernatants as lysates and determine total protein concentration with BCA assay (Pierce) using a small aliquot.[0199]6. Aliquot the rest for storage at −80° C. until use.
Assay Design:[0200]1. The total assay volume is 40 ul.[0201]2. The lysates are tested in serial titration series of 40, 20, 10, 5, 2.5, 1.25, 0.63, 0.31 ug total-equivalents and the volume...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com