Abietane type tricyclic diterpenoid C-14 site hydroxylase
A technology of tricyclic diterpenes and compounds, applied in the field of tripterygium wilfordii cytochrome P450 oxidase, can solve problems such as unknown multi-step pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
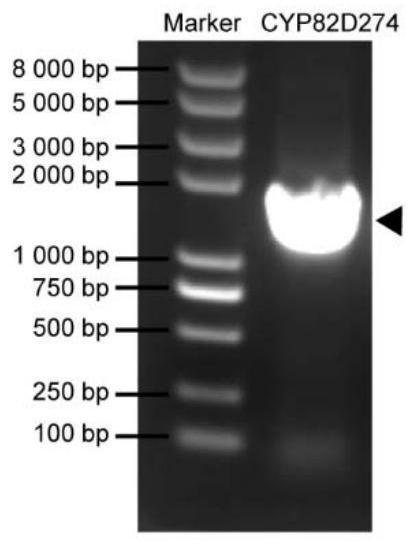
[0043] Embodiment 1, Tripterygium wilfordii CYP82D274 gene cloning ( figure 1 )
[0044] First, use Super Total RNA Extraction Kit (Promega) for operation:
[0045] (1) Take about 10 mg of Tripterygium wilfordii suspended cell sample and place it in a 2 mL centrifuge tube, pulverize it evenly in a liquid nitrogen environment, and quickly pulverize it with a mixing mill (MM400, Retsch) for 2 minutes;
[0046] (2) Quickly add 500 μL RNA Lysis Solution to the EP tube containing the pulverized sample, and invert the centrifuge tube 3-4 times to fully lyse the sample;
[0047] (3) Continue to add 500 μL RNA diluent, mix with a pipette, centrifuge at 14 000 rpm at 4°C for 5 min;
[0048] (4) Take the supernatant into a new sterile 2mL centrifuge tube, add 0.5 times the volume of absolute ethanol, and invert the centrifuge tube to fully mix;
[0049] (5) Transfer the mixed solution to a spin column in stages, centrifuge at 12 000 rpm at 4°C for 1 min, and discard the filtrate; ...
Embodiment 2
[0076] Example 2. In vivo functional characterization of CYP82D274 yeast
[0077] First, construct a eukaryotic expression vector containing P450 and CPR
[0078] (1) Construction of pESC-LEU::TwCPR3
[0079] Since the function of P450 requires cytochrome P450 oxidoreductase (CPR) to provide electrons, tripterygium wilfordii cytochrome P450 oxidoreductase 3 (TwCPR3) was constructed to the multiple cloning site MCS2 (multiple cloning site) terminal. The carrier part is subjected to double digestion reaction with NEB restriction endonucleases BamHI-HF and SalI-HF, and the reaction system is:
[0080]
[0081] After the reaction is over, perform electrophoresis detection together with the undigested carrier and 15K DNA Marker (full gold), and confirm that there is a band of the target size and the expression form is different from that of the undigested sample, and the digestion is considered successful. Use 1.5% agar Glycogel electrophoresis (150V, about 25min) and gel rec...
Embodiment 3
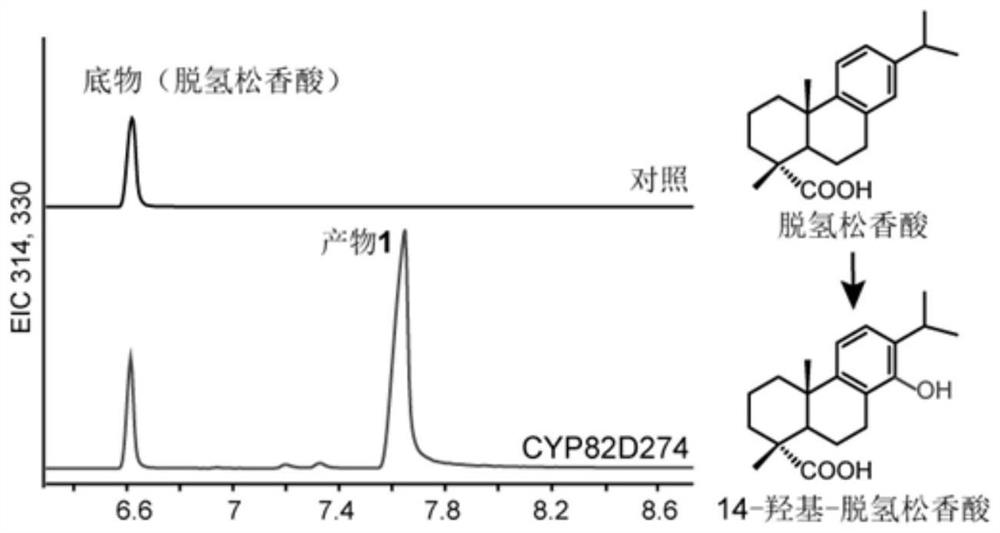
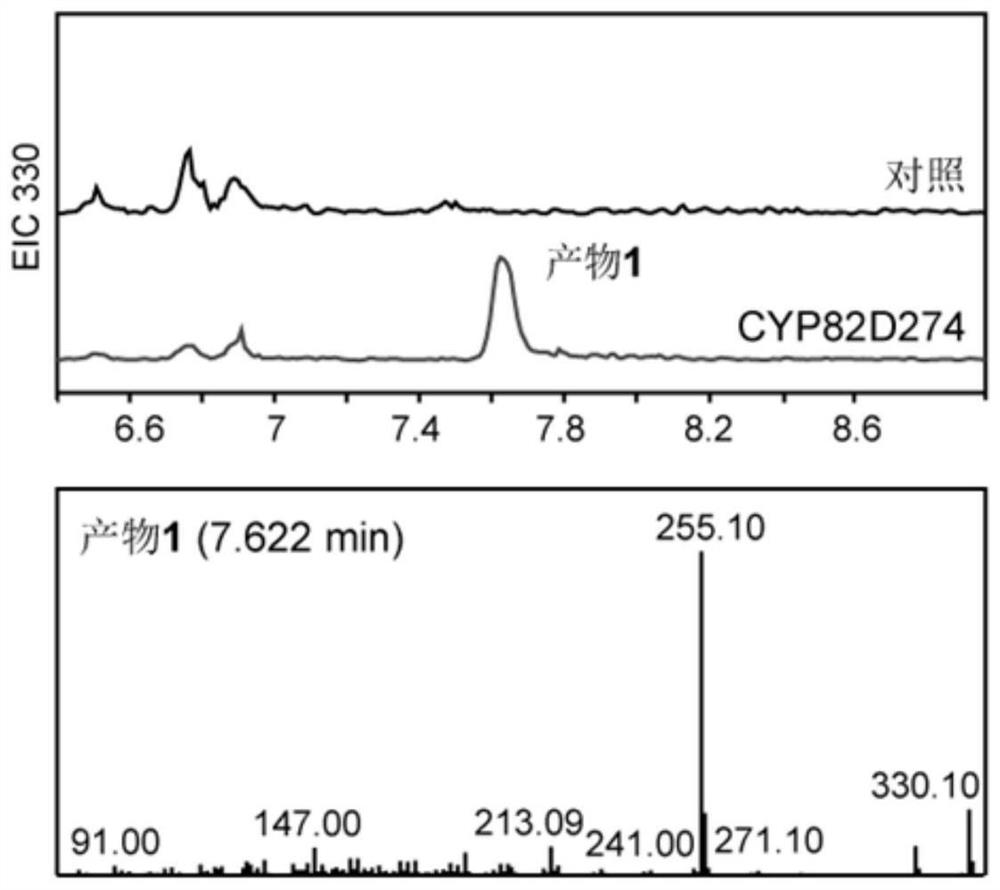
[0118] Example 3, In vitro functional characterization of CYP82D274 yeast ( image 3 )
[0119] Microsomes were extracted from the BY47471 Saccharomyces cerevisiae containing pESC-LEU::(CYP82D274+TwCPR3) and the corresponding empty vector pESC-LEU::TwCPR3, and dehydroabietic acid was used as a substrate to detect the product in vitro.
[0120] The microsome extraction method is as follows:
[0121] (1) Transfer the activated bacterial liquid into 50mL SD-Leu+2%Glc liquid medium, incubate at 200rpm at 30°C for 16-20h, collect the bacterial liquid, and centrifuge at 4000g for 5min at room temperature;
[0122] (2) Resuspend the cells with 100mL inducible SD-Leu+2%Gal liquid medium, induce at 30°C, 200rpm for 12-16h;
[0123] (3) Bacterial solution was centrifuged at 4 000 g for 3 min at 4°C to collect the bacterial cells, resuspended in 10 mL TEK solution (TE (50 mM Tris-HCl, 1 mM EDTA, pH 7.4) + potassium chloride KCl), and allowed to stand at room temperature 5min;
[0124...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com