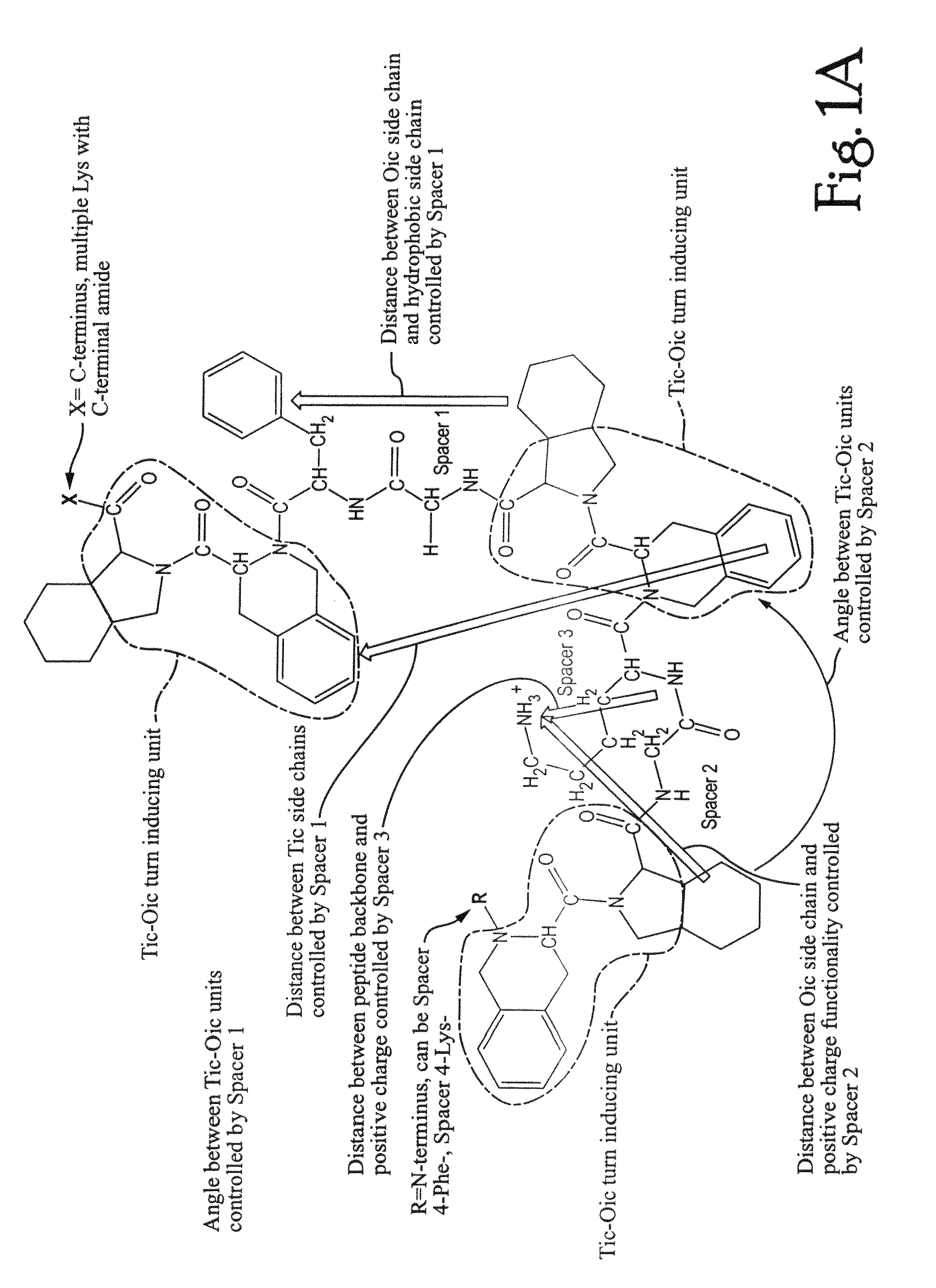
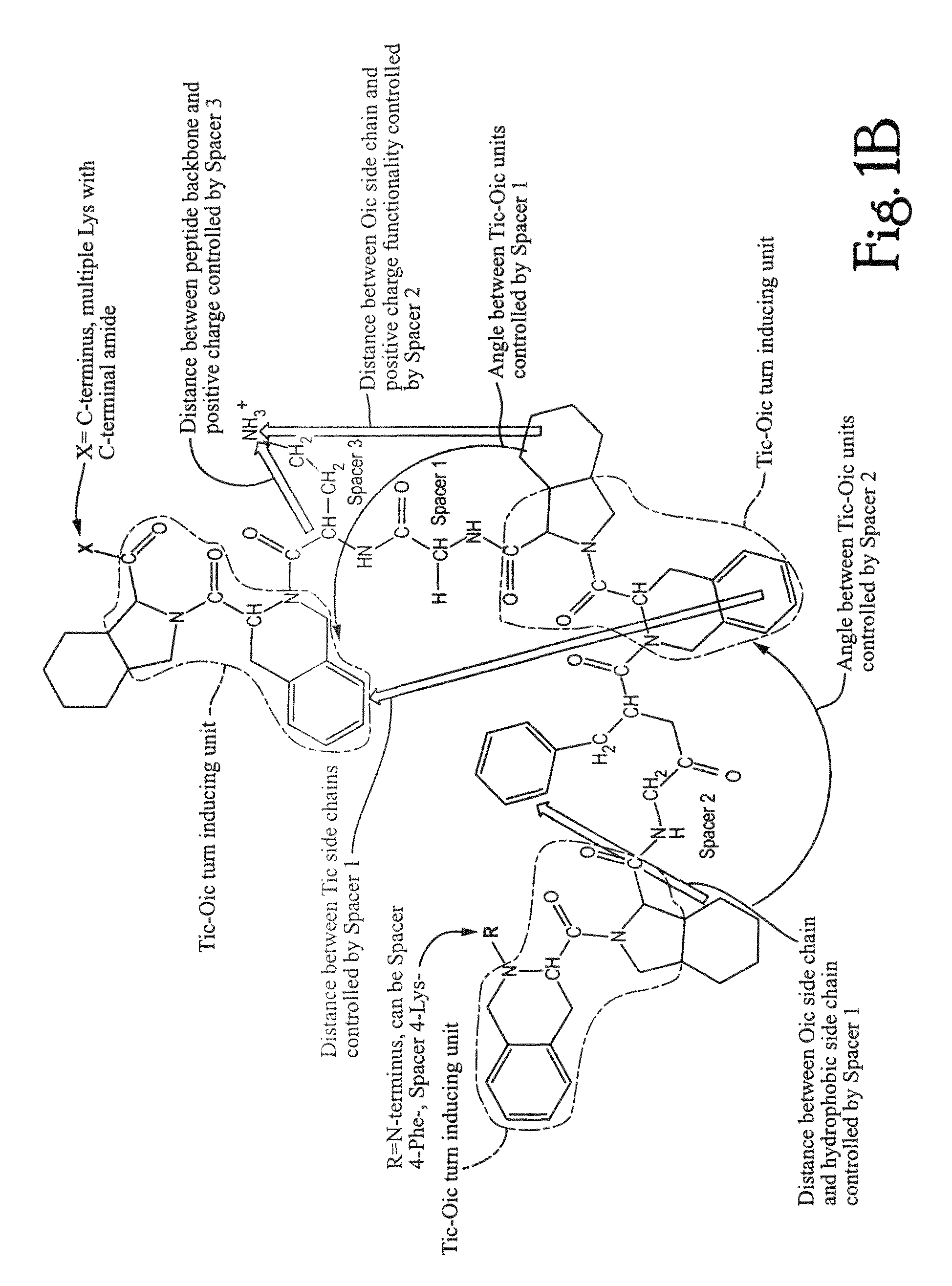
Anti-microbial peptidomimetic compounds and methods to calculate anti-microbial activity
a technology of peptidomimetic compounds and anti-microbial activity, applied in the field of new molecules, can solve the problems of general no longer accepting that amps are uniform and indiscriminate, and achieve the effect of providing conformational flexibility for the backbon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Antimicrobial Peptides
[0271]To develop effective synthetic AMPs, the inventors selected analogs of the magainin family of host defense peptides because these peptides are active against Gram positive and negative bacteria, fungi and protozoa while exhibiting little mammalian cell toxicity.24 The interactions of the magainins25, 26 with membrane models have been extensively investigated resulting in the characterization of the magainins as well defined α-helical amphipathic cell-selective membrane-disruptors.16, 18, 19
[0272]The inventors previously reported two-dimensional NMR and molecular modeling.27 Investigations conducted in their laboratory indicated that (Ala 8,13,18)magainin-2-amide bound to dodecylphosphocholine (DPC) micelles adopts a α-helical structure involving residues 2 to 16 with the four C-terminal residues converging to a loose β-turn like structure. While (Ala8,13,18)magainin-2-amide bound to sodium dodecylsulfate (SDS) micelles adopts a α-helical structure involv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angles | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com