Process of synthesis of 3',4'-anhydrovinblastine, vinblastine and vincristine
a technology of vinblastine and vincristine, which is applied in the field of synthesis of dimer alkaloid compounds, can solve the problems that art methods also yield small quantities of target compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Iminium Intermediate (Formula VI) via Modified Polonovski Reaction
The reaction was performed under anhydrous conditions. All glassware was oven-dried at 120.degree. C. The solvent, methylene chloride, and coupling reagent, trifluoroacetic anhydride, were distilled from P.sub.2 O.sub.5 prior to use.
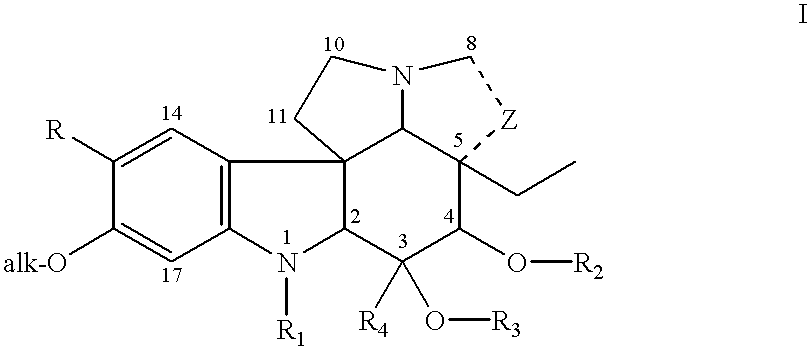
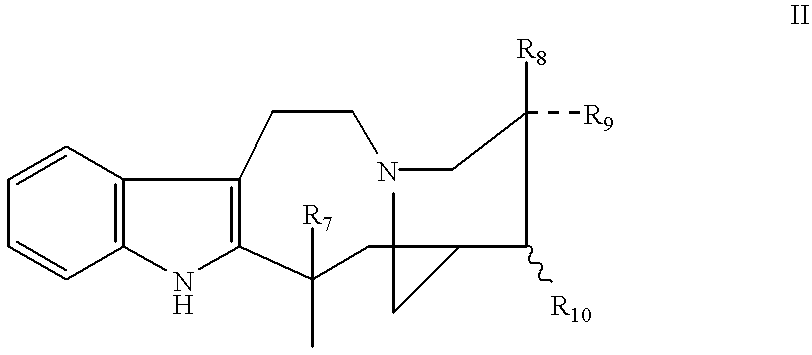
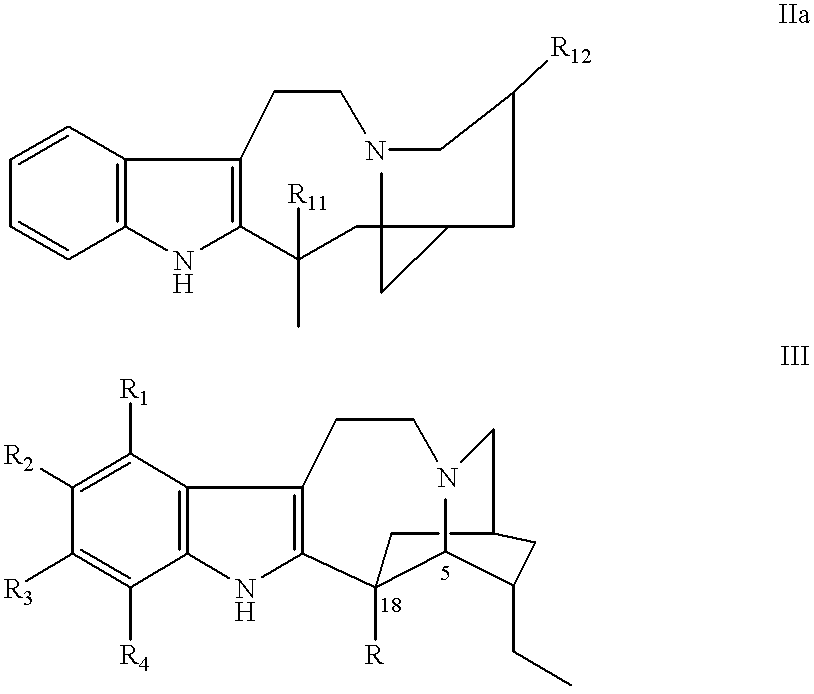
To a solution of catharanthine (Formula III, 200 mg, 0.6 mmol) in dry methylene chloride (2 ml) at -20.degree. C. under a positive atmosphere of argon was added m-chloroperbenzoic acid (132 mg, 0.8 mmol), and the mixture was stirred for 5 minutes. To the catharanthine N-oxide (IIIa; R.dbd.COOCH.sub.3 ; R.sub.1, R.sub.2, R.sub.3, and R.sub.4 =H), thus formed was added a solution of vindoline (IV, 270 mg, 0.6 mmol) in methylene chloride (1 ml) and the mixture was cooled to -60.degree. C. Trifluoroacetic anhydride (0.2 ml, 1.5 mmol) was added to the stirred reaction mixture maintained at -60.degree. C. for 2 hours. After this time, the solvent and excess reagents were remov...
example 2
Reduction of Iminium Intermediate (Formula VI) with 1-Benzyl-1,4-dihydronicotinamide [Formula IX, R.sub.1 =benzyl, R.sub.2, R.sub.4, R.sub.5, and R.sub.6 =H; R.sub.3 =CONH.sub.2 ; (Formula IX-A) Procedure A]
To a stirred solution of iminium intermediate (VI, 100 mg) in degassed acetonitrile (5 ml) was added 1-benzyl-1,4-dihydronicotamide (135 mg, 0.63 mmol, 6 equivalents) under a positive atmosphere of argon (greater than 760 mm Hg) at room temperature (20.degree. C.) over a period of 5 hours. After this time, the reaction mixture, as monitored by reverse phase HPLC (Waters Radial-Pak C.sub.18 or CN cartridge, methanol / H.sub.2 O / Dt.sub.3 N solvent system), indicated complete conversion of VI to a mixture of enamine VIII and 3',4'-dehydrovinblastine (VII) in a ratio of 1:1 (75% yield).
example 3
Reduction of Iminium Intermediate (Formula VI) with 1-Benzyl-1,4-dihydronicotinamide (Formula IX, R.sub.1 =benzyl, R.sub.2, R.sub.4, R.sub.5, and R.sub.6 =H; R.sub.3 =CONH.sub.2 (Formula IX-A) Procedure A]
To a stirred solution of iminium intermediate (VI, 100 mg) in methanol (5 ml) kept initially at 0.degree. C for 0.5 hours was added dropwise or in portions, a solution of 1-benzyl-1,4-dihydronicotinamide (56 mg, 0.26 mmol, 2.5 equivalents) in methanol (2ml) under a positive atmosphere of argon (greater than 760 mm Hg) over a period of 5 hours. During this time the solution was allowed to warn up to room temperature. HPLC monitoring, as in Example 1, indicated complete conversation of VI to a mixture of enamine VIII and 3',4'-dehydrovinblastine (VII) in a ratio of 1:1 (75% yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com