Pulse oximetry testing
a pulse oximeter and oximeter technology, applied in the field of pulse oximeter testing, can solve the problems of inability to fully test, inaccurate readings, abuse, etc., and achieve the effect of eliminating doubts about integrity and facilitating testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
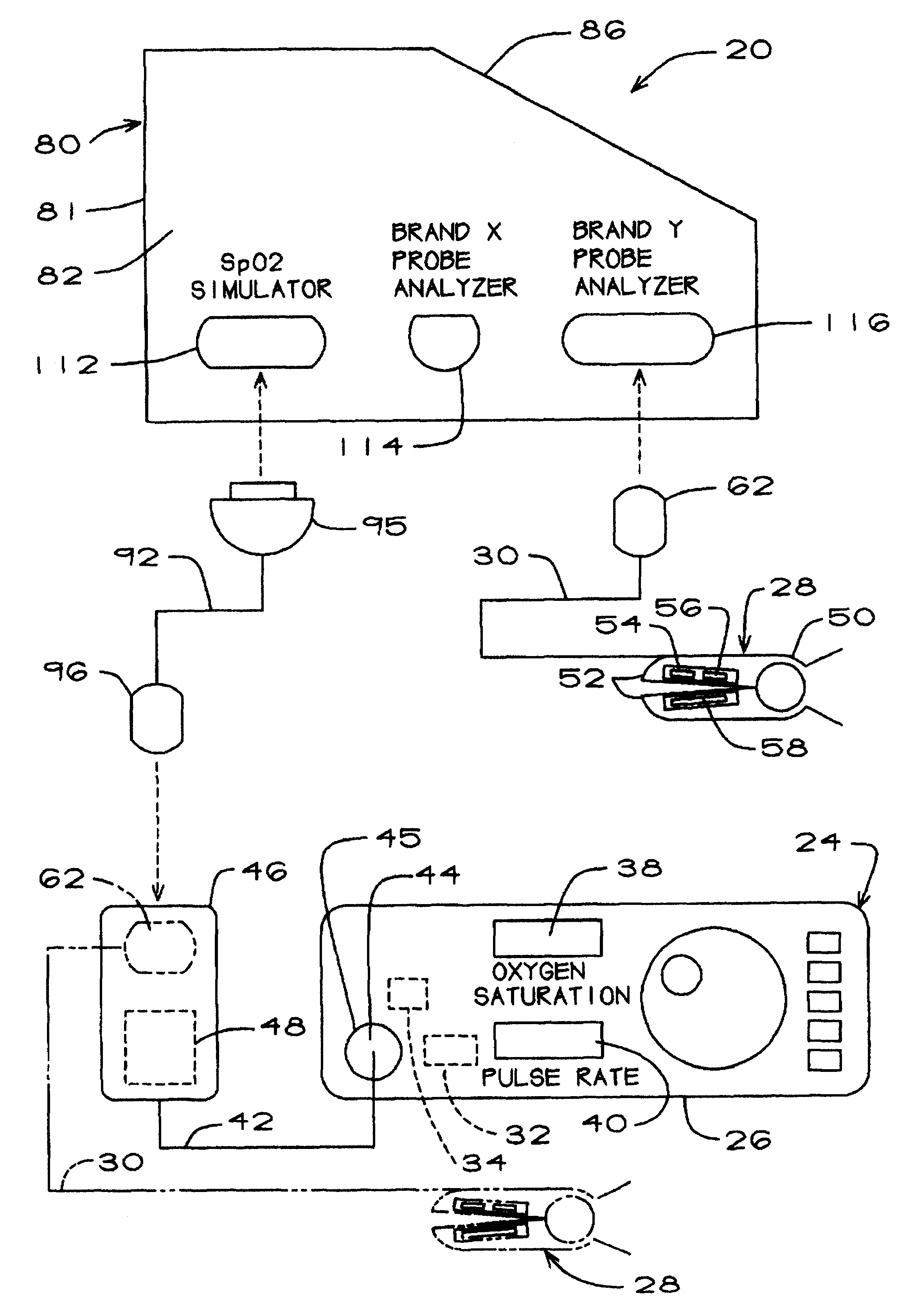
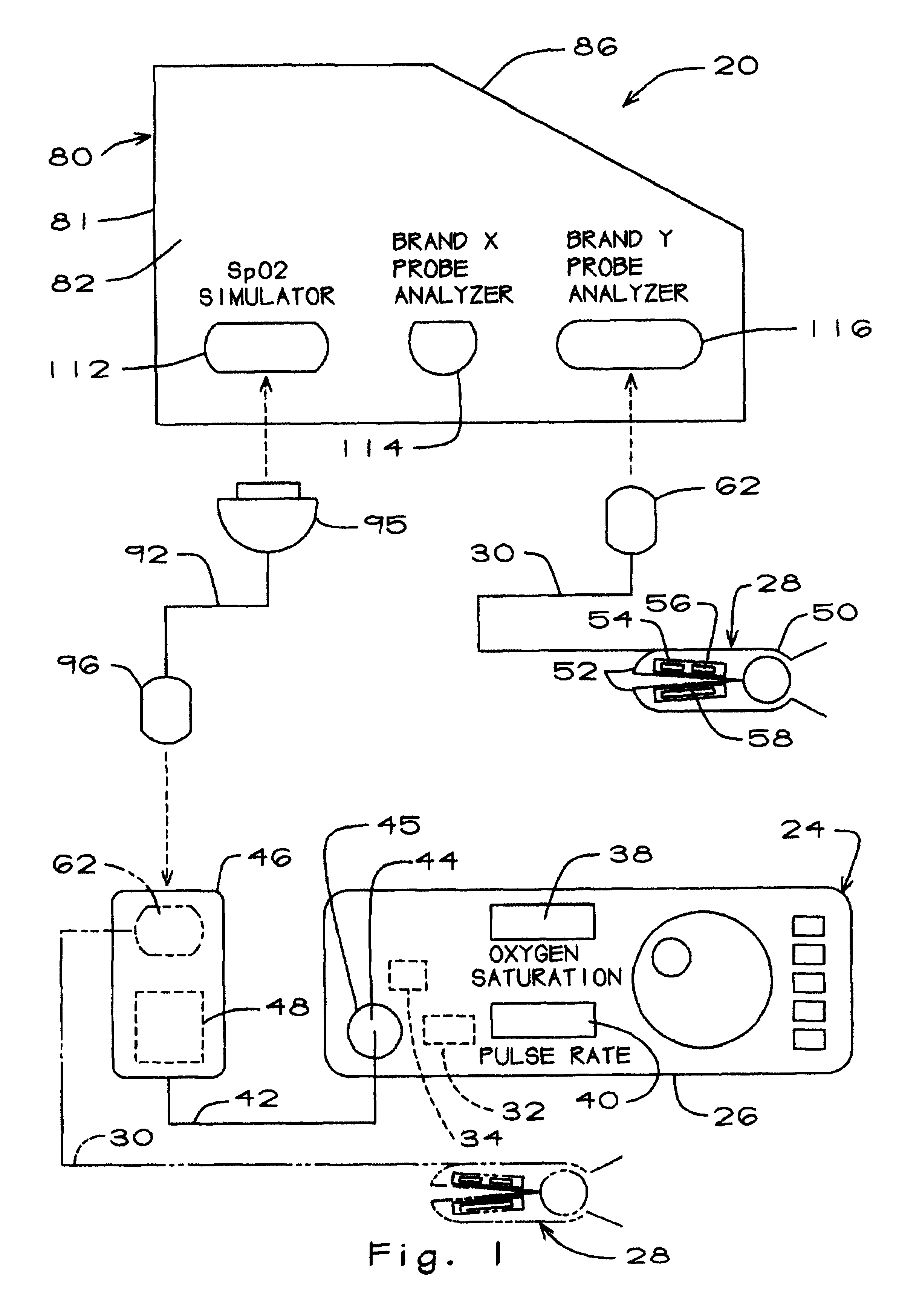
The pulse oximeter testing apparatus or analyzer of the present invention is generally represented by the numeral 20 in FIG. 1, which is a schematic drawing showing how the testing apparatus is connected to a pulse oximeter 24. Since reference will made herein to certain aspects of the pulse oximeter itself, it will be helpful at the outset to provide a brief general description of a typical pulse oximeter.
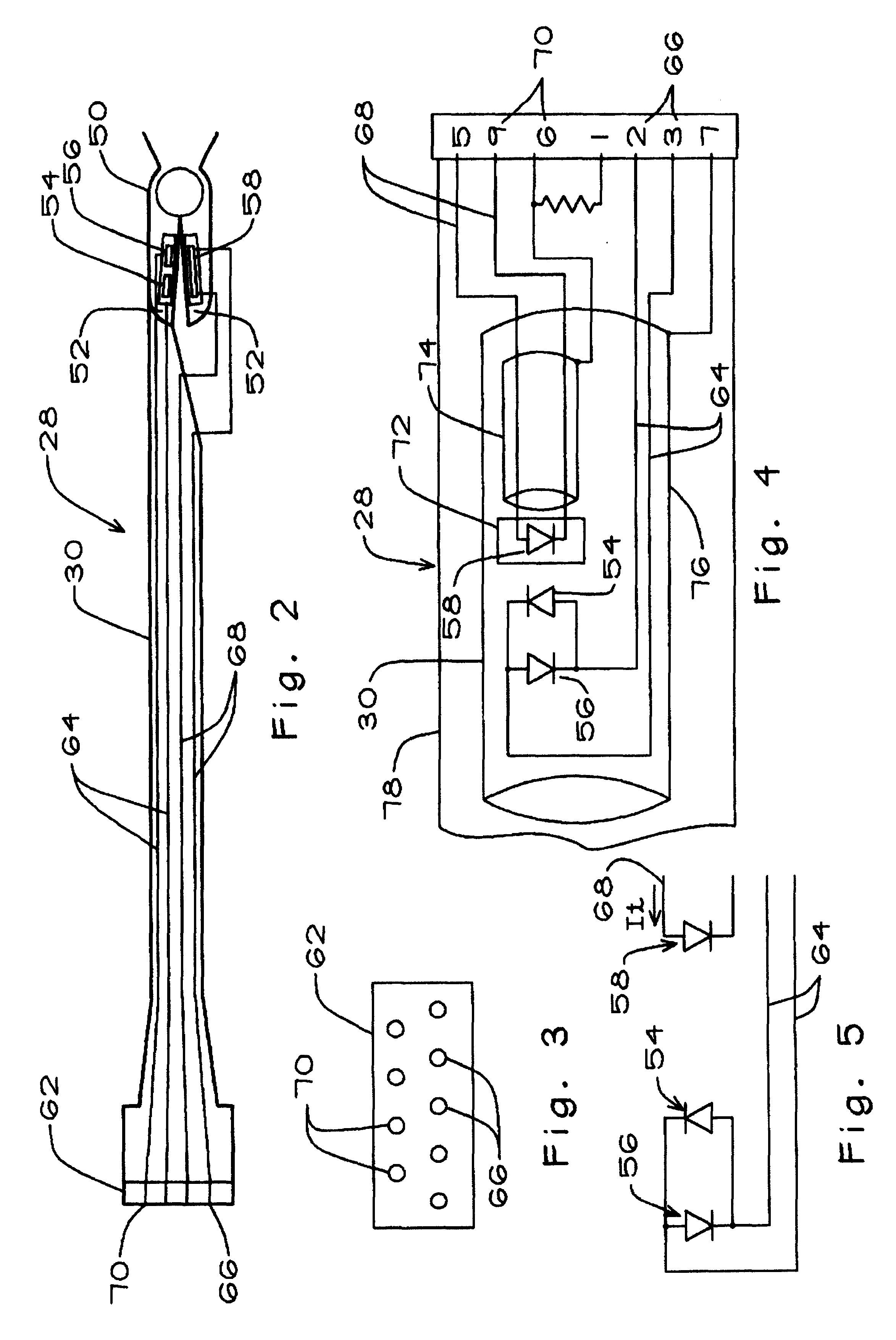
Thus, the pulse oximeter 24 (FIG. 1) includes a main unit 26 and a probe 28 which includes a probe cable 30. The main unit includes a signal processor 32, an LED driver 34, and upper and lower displays 38 and 40 for displaying the blood oxygen saturation SpO.sub.2 as a percentage and pulse rate in beats per minute. The main unit of the brand of oximeter shown and described herein also includes a preamp cable 42. The preamp cable terminates at one end in a proximal preamp connector 44 coupled to an interface connector 45 in the main unit 26 and at the opposite end in a distal. prea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| electrical continuity | aaaaa | aaaaa |
| electrical sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com