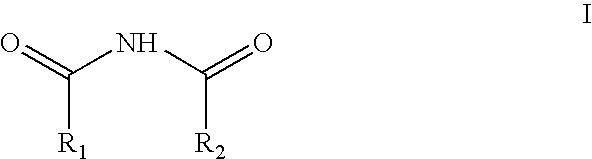
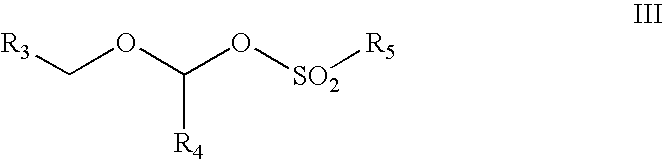
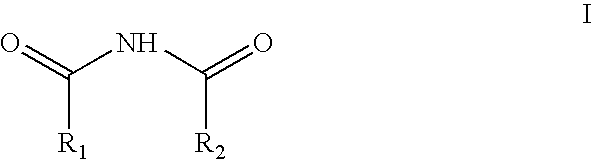
Method and reagents for N-alkylating ureides
a technology of n-alkylated ureides and reagents, which is applied in the direction of biocide, drug compositions, muscular disorders, etc., can solve the problems of complex reaction, high toxic and regulated, salts, dimers, and other side products being formed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N,N′-bismethoxymethyl-5,5-diphenylbarbituric acid
A. Using a Hindered Tertiary Amine Catalyst
[0044]Dimethoxymethane (10.85 g) was added at 0° C. to 19.7 g of acetylmethanesulfonate. The reaction was stirred at 25° C. for 2 hours. The resultant solution was then added gradually over 45 minutes to a mixture of 10 g of 5,5-diphenylbarbituric acid and 13.85 g of N,N-diisopropyl ethyl amine in 60 ml of dry dimethylformamide. The resultant reaction mixture was stirred for about 15 minutes and then diluted with 180 ml of 2 N HCl, followed by 300 ml of ethyl acetate. The phases were separated and the ethyl acetate phase was washed first with 150 ml of saturated aqueous sodium chloride and then with 150 ml of 2N aqueous NaOH. The organic (ethyl acetate) phase was dried over anhydrous sodium sulfate, filtered and concentrated to dryness to give 12.2 g of N,N′-bismethoxymethyl-5,5-diphenylbarbituric acid. Crystalization from 48 ml of toluene afforded 10.5 g of pure product (79.6% yield).
B. Usin...
example 2
N,N′-Bisethoxymethyl-5,5-diphenylbarbituric acid
[0049]By the procedure of Example 1A, using diethoxymethane (15.42 g) in place of dimethoxymethane (10.85 g), there is obtained a 68% yield of pure N,N′-bismethoxymethyl-5,5-diphenylbarbituric acid.
example 3
3-Methoxymethylphenytoin
[0050]By the procedure of 1A, using phenytoin (18 g) in place of 5,5-diphenylbarbituric acid (10 g), there is obtained a 70% yield of 3-methoxymethylphenytoin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| muscle stiffness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com