Process for removing mercury from flue gases
a technology of flue gas and mercury, which is applied in the direction of separation processes, dispersed particle separation, chemistry apparatus and processes, etc., can solve the problems of high additional capital costs, insufficient effectiveness of previous techniques for reducing mercury, and restrictive limiting values for legally permissible mercury emission, etc., to suppress free halogens and increase consumption of sulphur dioxid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
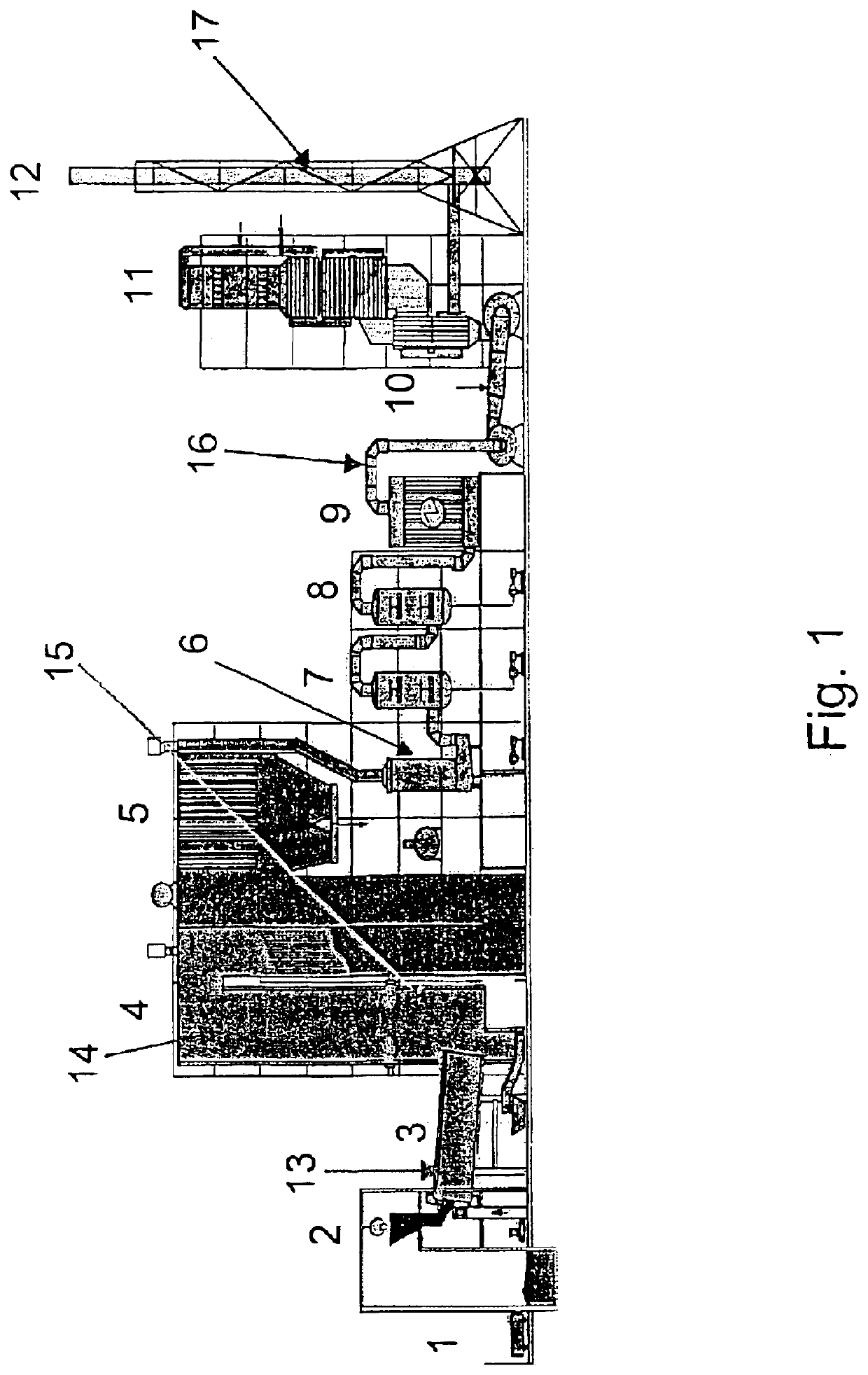
[0034]Examples 1-4 have been carried out in a special hazardous incineration plant of Bayer AG in Leverkusen corresponding to the diagram in FIG. 1. The rotary kiln 3 as primary combustion chamber is fired with solid waste from the bunker 1 via a crane grab 2, with liquid waste from a liquid waste tank and with waste drams via a dram feeder. The afterburning chamber 4, as a secondary combustion chamber, is also fired with liquid waste. The flue gas is cooled via the waste-heat boiler 5 and then, as what is termed dirty boiler gas, fed to the wet flue gas emission control system (multistage scrubber), which encompasses a quench 6, an acid rotary atomizer scrubber 7, an alkaline rotary atomizer scrubber 8 and an electrostatic gas cleanup system involving partial condensation of steam 9. Via suction fans 10 the scrubbed dirty gas, as what is termed clean gas, passes into the downstream catalytic denitrification plant 11 (selective catalytic denitrification of the clean gas by means of ...
example 1
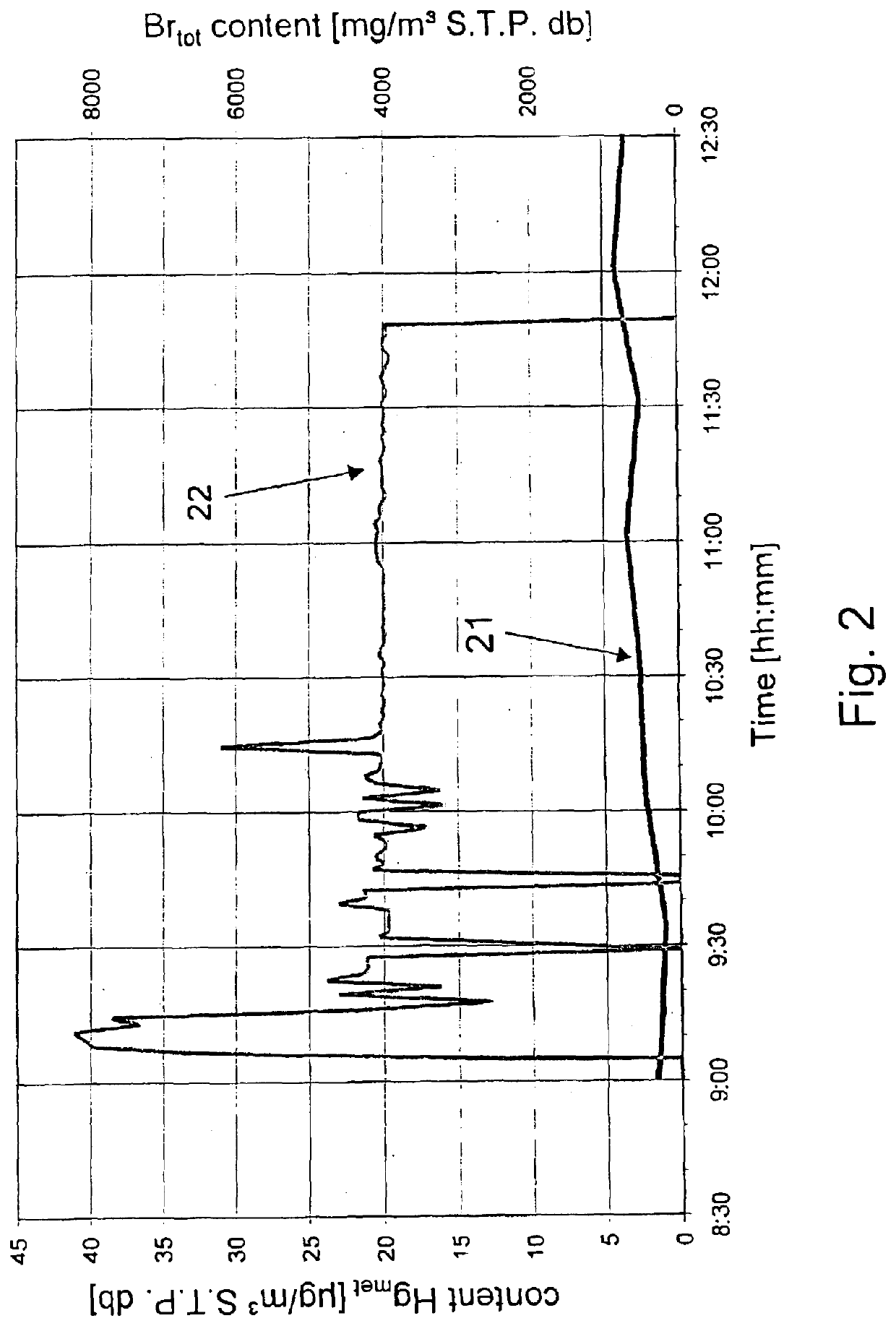
[0036]Over a period of 116 minutes, a series of samples of metallic mercury in plastic capsules (in total 3400 g, see Table 1) were fed to the secondary combustion chamber (afterburning chamber 4) via the inspection port 15. The feed was performed at intervals of approximately 5-10 minutes with increasing amount of mercury. The mercury introduced vaporizes within approximately 2-4 minutes; therefore, the instantaneous peak mercury concentrations occurring in the boiler flue gas at a volume flow rate of approximately 45·103 m3 S.T.P. db / h can be estimated. The estimation at the end of the experiment gives peak mercury concentrations of more than 130·103 μg / m3 S.T.P. db.
[0037]
TABLE 1Addition of Hg samplesTimeHg amount [g]TimeHg amount [g] 9:24 510:32180 9:32 1010:37200 9:38 1510:43220 9:49 2010:48240 9:54 4010:53260 9:59 6010:5828010:04 8011:0330010:0910011:0831010:1512011:1332010:2014011:2034010:26160Experimental time [min]Total Hg amount [g]1163400
[0038]During the experimental perio...
example 2
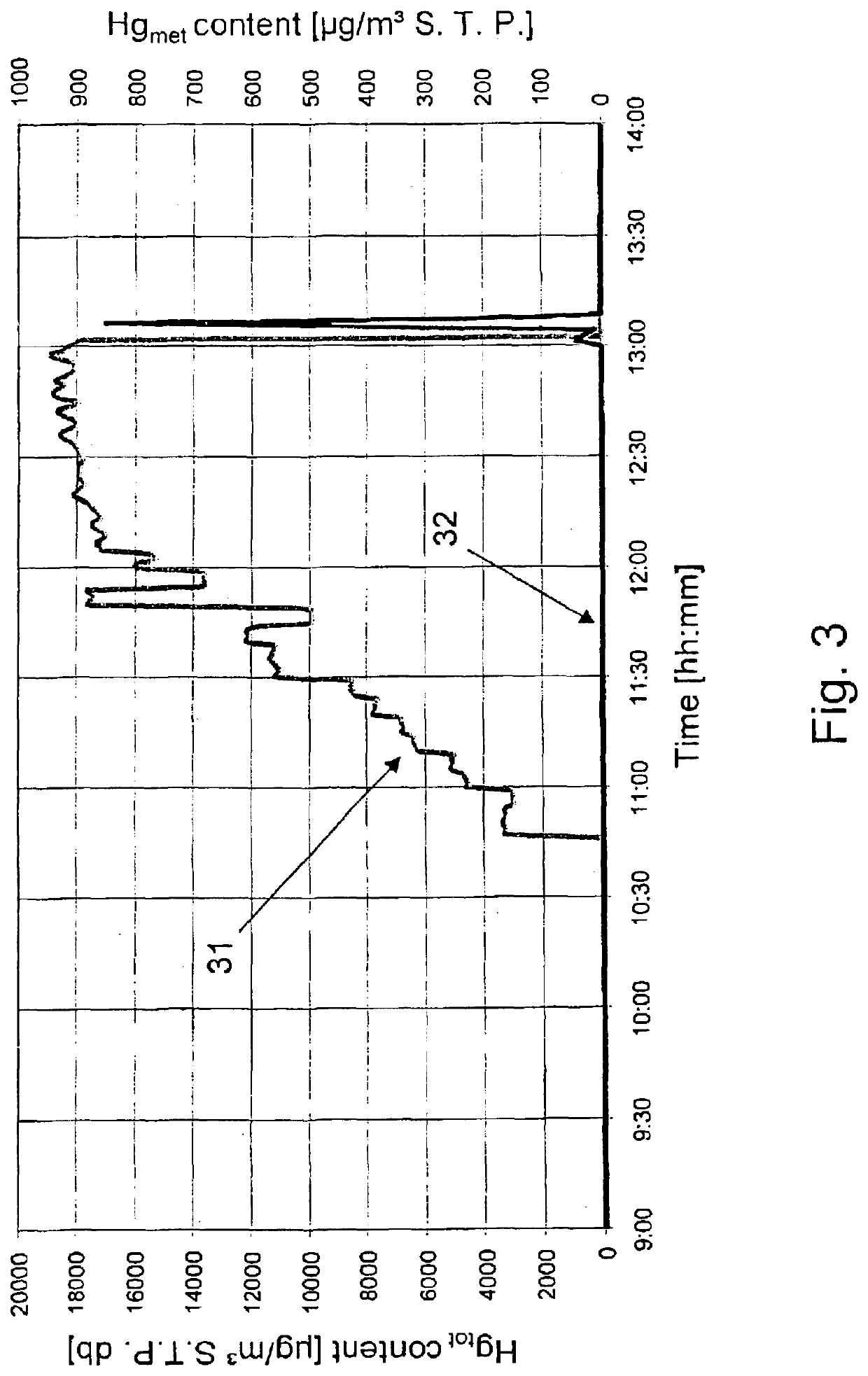
[0041]Over a period of 130 minutes, an aqueous HgCl2 solution was fed continuously to the secondary combustion chamber (afterburning chamber 4) via a nozzle in the afterburning chamber roof. The rate added was increased here at intervals of about 5 minutes. FIG. 3 shows the increase in mercury concentration thus induced in the boiler flue gas in the time between approximately 10:45 and 13:00. The mercury introduced is immediately released in the afterburning chamber as metallic mercury Hgmet. The total mercury concentration in the boiler flue gas increased in this manner to values of 18·103 μg / m3 S.T.P db (curve 31 and left y axis). The Hg concentration in the boiler flue gas was calculated from the mercury addition rate and the flue gas volume flow rate measured operationally. During the experimental period, by co-incineration of a highly brominated liquid waste (addition via a burner at the rotary kiln head) a bromine content of approximately 9·103 mg / m3 S.T.P. db was maintained i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com