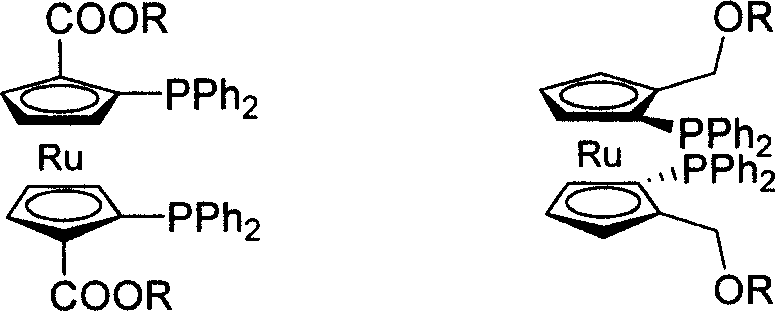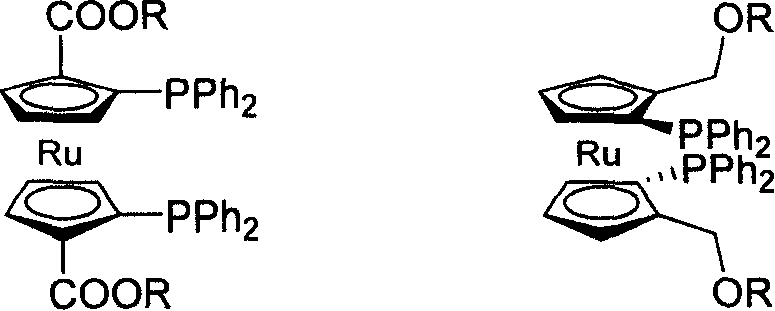C2-symmetrical bis ruthenium Diphosphine Ligand only with surface chirality
A bisphosphine ligand and chirality technology, which is applied in the ligand field of the chemical technology field, can solve the problems such as the same or similar literature reports that have not been found, and achieve the effects of high reactivity, stereoselectivity, and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 1, the preparation of amide ester (2)
[0016] (S)-(S)-1,1'-bis(diphenylphosphino)-2,2'-bis[(S)-4-isopropyloxazolinyl]ruthenocene (1.65g, 2mmol) was dissolved in tetrahydrofuran (40mL), then water (2mL), trifluoroacetic acid (3.8mL, 49.4mmol), anhydrous sodium sulfate Na 2 SO 4 (18.8g), the suspension was stirred overnight at room temperature, filtered, the solvent was evaporated, the residue was dissolved in dichloromethane (40mL) and then pyridine (7.2mL, 89mmol) and acetic anhydride (12.0mL, 76.4mmol) were added successively, and stirred at room temperature Overnight, the mixture was diluted with dichloromethane (80 mL), washed with dilute hydrochloric acid (10%), water, and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated to obtain 1.65 g of the target product, y=82.8%.
[0017] 1 H NMR (400MHz, CDCl 3 ): δ7.34-7.14 (m, 20H, ArH), 6.59-6.58 (brs, 2H, NH), 5.39 (brs, 2H, RcH), 4.83 (brs, 2H, RcH), 4.39-4.34 (dd, J=4Hz, 15.6...
Embodiment 2
[0022] 1, the preparation of amide ester (2)
[0023] (S)-(S)-1,1'-bis(diphenylphosphino)-2,2'-bis[(S)-4-isopropyloxazolinyl]ruthenocene (1.65g, 2mmol) was dissolved in tetrahydrofuran (40mL), then water (2mL), trifluoroacetic acid (3.8mL, 49.4mmol), anhydrous sodium sulfate Na 2 SO 4 (18.8g), the suspension was stirred overnight at room temperature, filtered, the solvent was evaporated, the residue was dissolved in dichloromethane (40mL) and then pyridine (7.2mL, 89mmol) and acetic anhydride (12.0mL, 76.4mmol) were added successively, and stirred at room temperature Overnight, the mixture was diluted with dichloromethane (80 mL), washed with dilute hydrochloric acid (10%), water, and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated to obtain 1.65 g of the target product, y=82.8%.
[0024] 1 H NMR (400MHz, CDCl 3 ): δ7.34-7.14 (m, 20H, ArH), 6.59-6.58 (brs, 2H, NH), 5.39 (brs, 2H, RcH), 4.83 (brs, 2H, RcH), 4.39-4.34 (dd, J=4Hz, 15.6...
Embodiment 3
[0029] 1, the preparation of amide ester (2)
[0030] (S)-(S)-1,1'-bis(diphenylphosphino)-2,2'-bis[(S)-4-isopropyloxazolinyl]ruthenocene (1.65g, 2mmol) was dissolved in tetrahydrofuran (40mL), then water (2mL), trifluoroacetic acid (3.8mL, 49.4mmol), anhydrous sodium sulfate Na 2 SO 4(18.8g), the suspension was stirred overnight at room temperature, filtered, the solvent was evaporated, the residue was dissolved in dichloromethane (40mL) and then pyridine (7.2mL, 89mmol) and acetic anhydride (12.0mL, 76.4mmol) were added successively, and stirred at room temperature Overnight, the mixture was diluted with dichloromethane (80 mL), washed with dilute hydrochloric acid (10%), water, and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated to obtain 1.65 g of the target product, y=82.8%.
[0031] 1 H NMR (400MHz, CDCl 3 ): δ7.34-7.14 (m, 20H, ArH), 6.59-6.58 (brs, 2H, NH), 5.39 (brs, 2H, RcH), 4.83 (brs, 2H, RcH), 4.39-4.34 (dd, J=4Hz, 15.6,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com