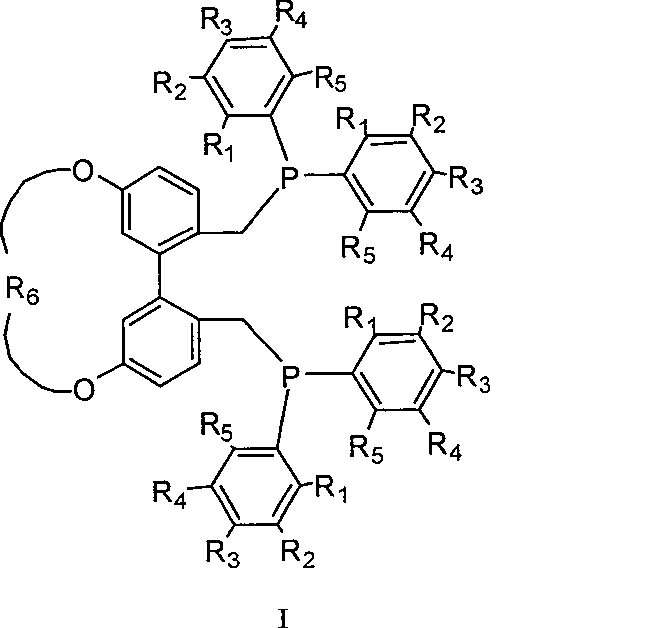
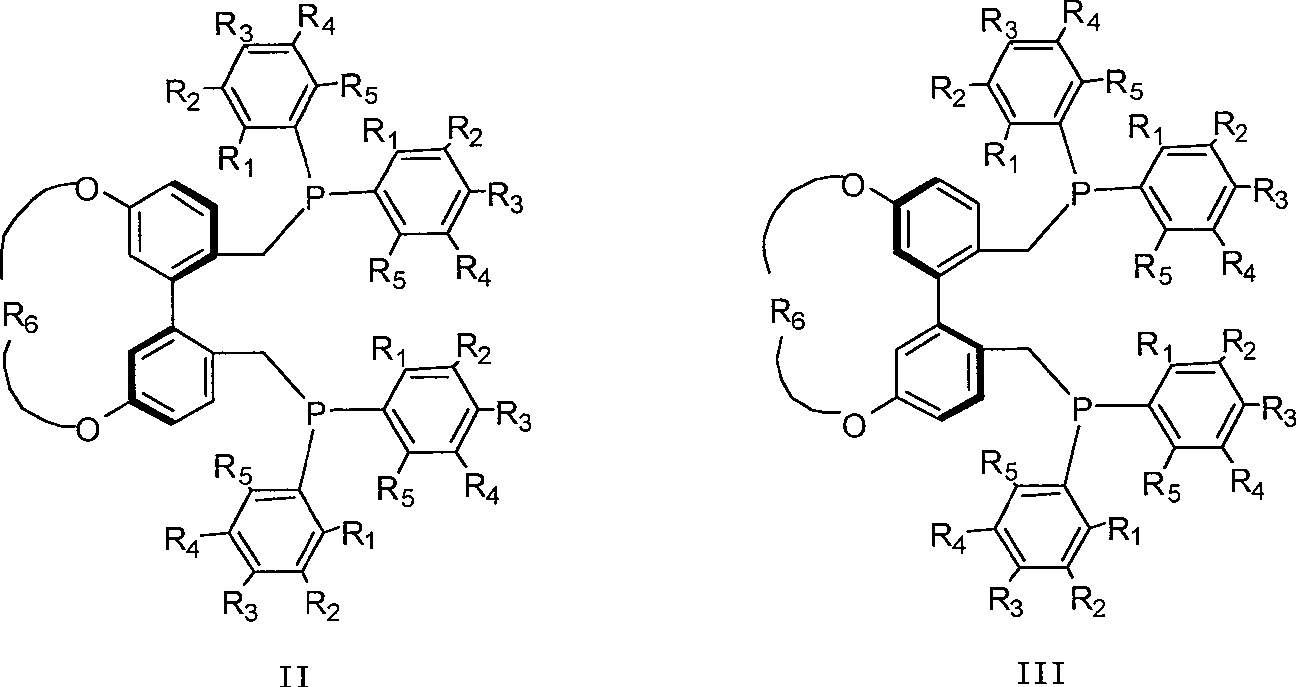
5,5'-position connected 1,1'-biphenyl axis chirality diphosphine ligand and synthetic method thereof
A technology of bisphosphine ligands and synthesis methods, applied in the field of 1, compounds and their synthesis, can solve problems such as limitations and achieve high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
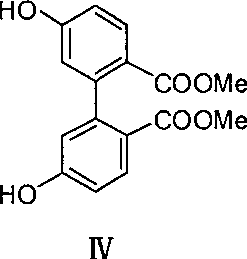
[0040] (1) Preparation of compound V from compound IV
[0041] Compound IV (1.0 g, 3.3 mmol), anhydrous potassium carbonate (1.4 g, 9.9 mmol) and 1,8-octanedibromide (0.6 mL, 3.3 mmol) were added to 200 mL N,N-dimethylformamide solution , stirred at room temperature for 72h. The reaction solution was filtered, N,N-dimethylformamide was aliquoted under reduced pressure, and the product V (0.8 g, 45%) was obtained by passing through the column with ethyl acetate and petroleum ether.
[0042] 1 H NMR (CDCl 3 , 400MHz) 7.94 (d, J = 8.4Hz, 2H, Ar-H), 6.91 (dd, J = 8.4, 2.4Hz, 2H, Ar-H), 6.76 (d, J = 2.4Hz, 2H, Ar- H), 4.40(t, J=7.6Hz, 2H, OCH), 4.37(t, J=7.6Hz, 2H, OCH), 3.62(s, 6H, OCH 3 ), 1.89-2.01 (m, 2H, CH 2 ), 1.55-1.67 (m, 2H, CH 2 ), 1.20-1.53 (m, 8H, CH 2 ).
[0043] (2) Preparation of compound VI from compound V
[0044] Compound V (0.3g, 1.1mmol) was dissolved in refined tetrahydrofuran solution, and anhydrous tetrahydrofuran solution of lithium aluminum hydr...
Embodiment 2
[0056] (1) Preparation of compound V from compound IV
[0057] Compound IV (0.8g, 2.6mmol), anhydrous potassium carbonate (1.1g, 7.9mmol) and 1,8-octanedibromide (0.5mL, 2.7mmol) were added to 180mL acetonitrile solution, stirred under reflux for 20h . The reaction solution was filtered, the acetonitrile was distilled off under reduced pressure, and the product V (0.7 g, 60%) was obtained by passing through the column with ethyl acetate and petroleum ether.
[0058] (2) Preparation of compound VI from compound V
[0059] Compound V (0.3g, 1.1mmol) was dissolved in refined tetrahydrofuran solution, and anhydrous tetrahydrofuran solution of lithium aluminum hydride (0.2g, 5.5mmol) was added dropwise under nitrogen atmosphere in an ice-water bath, and the reaction was carried out under reflux for 12 hours. Extract with saturated sodium sulfate. Acidification with dilute hydrochloric acid, extraction with ethyl acetate, MgSO 4 dry. The product VI (0.26 g, 100%) was obtained. ...
Embodiment 3
[0067] (1) Preparation of compound V from compound IV
[0068]Compound IV (0.8g, 2.6mmol), anhydrous potassium carbonate (1.1g, 7.9mmol) and 1,8-octanedibromide (0.7mL, 3.9mmol) were added to 200mL acetone solution, stirred under reflux for 12h . The reaction solution was filtered, the acetonitrile was distilled off under reduced pressure, and the product V (0.6 g, 51%) was obtained by passing through the column with ethyl acetate and petroleum ether.
[0069] (2) Preparation of compound VI from compound V
[0070] Compound V (0.3g, 1.1mmol) was dissolved in refined tetrahydrofuran solution, and anhydrous tetrahydrofuran solution of lithium aluminum hydride (0.1g, 2.8mmol) was added dropwise under nitrogen atmosphere in an ice-water bath, and the reaction was refluxed for 24 hours. Extract with saturated sodium sulfate. Acidification with dilute hydrochloric acid, extraction with ethyl acetate, MgSO 4 dry. The product VI (0.25 g, 95%) was obtained.
[0071] (3) Compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com