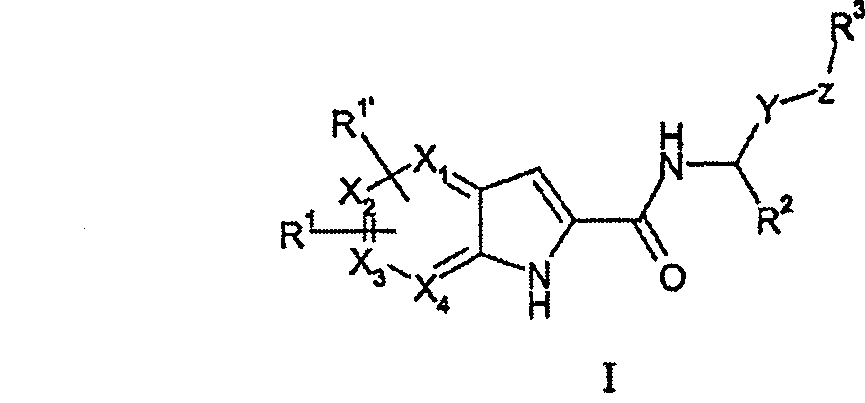
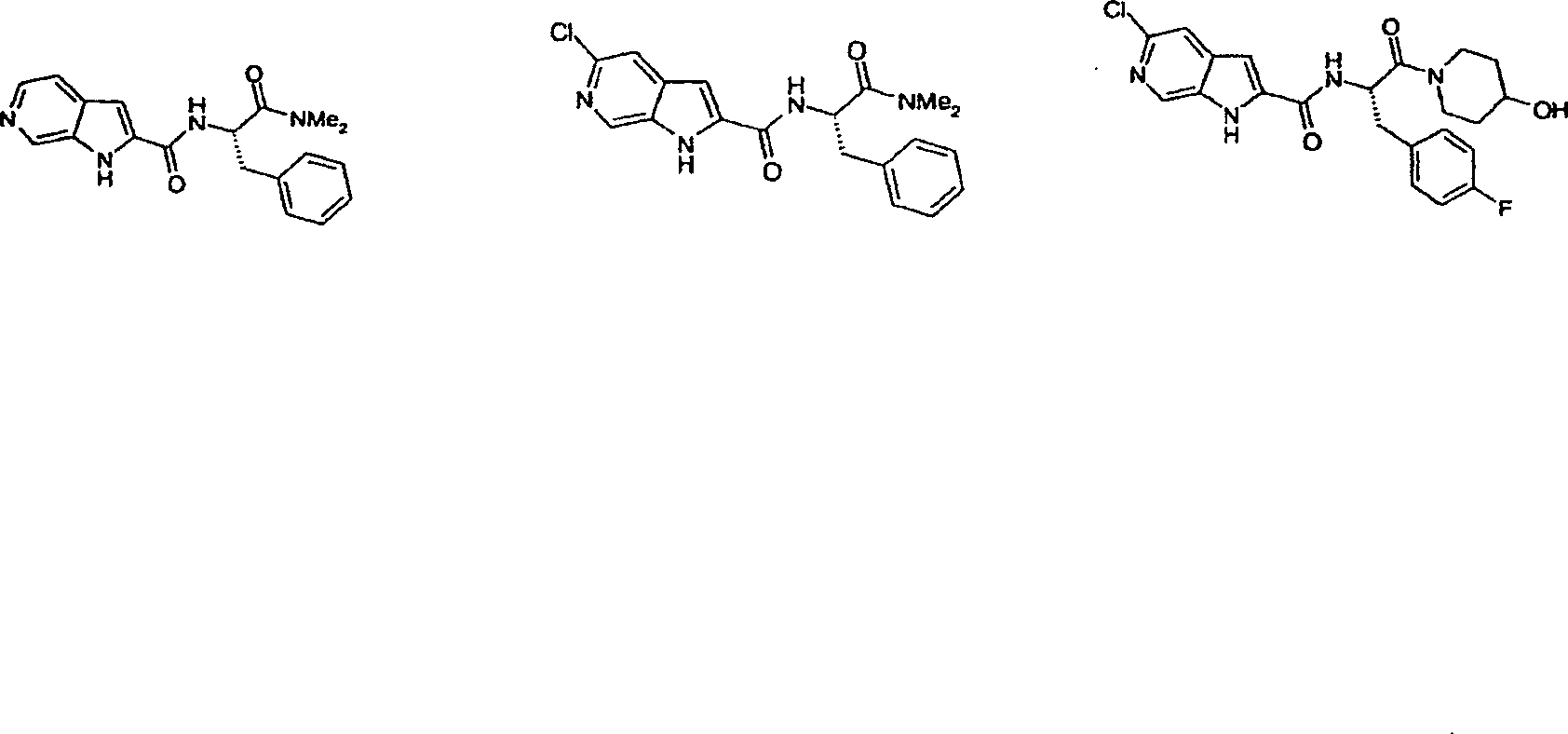
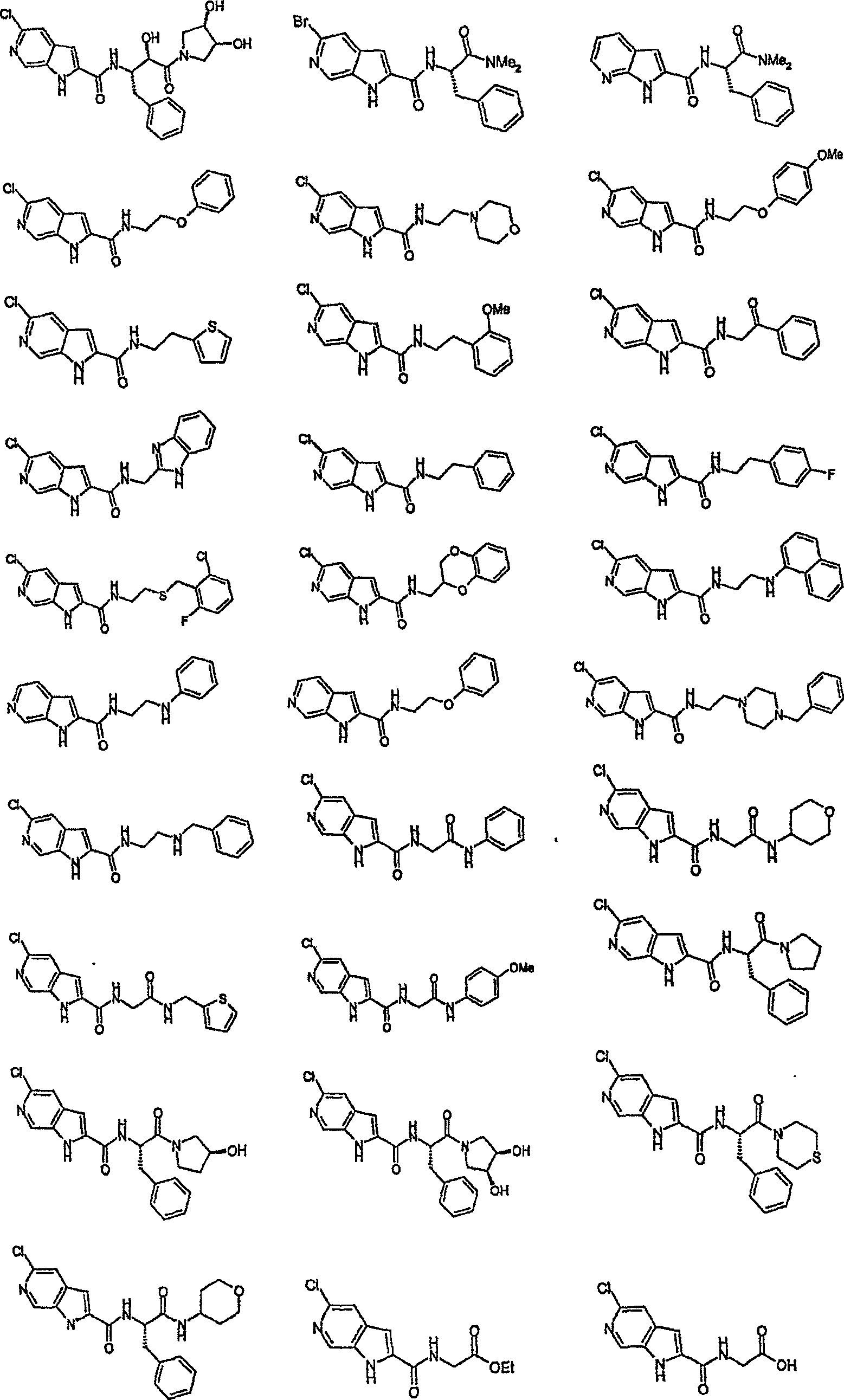
Pyrrolopyridine-2-carboxylic acid amide inhibitors of glycogen phoshorylase
A compound, trifluoromethyl technology, applied in the field of pyrrolopyridine-2-carboxamide, can solve the problem of difficult treatment of non-insulin-dependent type II diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0131] According to the present invention, compounds of general formula (I) can be prepared as outlined in Scheme 1 below, wherein R1 , R 1’ , R 2 , R 3 、X 1 、X 2 、X 3 、X 4 , Y and Z are as above-mentioned definition to general formula (I):
[0132] Route 1:
[0133]
[0134] According to Scheme 1, compounds of general formula (I) can be prepared by reacting an appropriate pyrrolopyridine-2-carboxylic acid of general formula (II) or a protected or activated derivative thereof with an appropriate amine of general formula (III). Coupled to prepare. Compounds of general formula (II) can be obtained by the synthesis described in Scheme 3 and Scheme 5 below. Compounds of general formula (III) are generally commercially available or may be obtained by the syntheses described in schemes 8 and 9 .
[0135] Typically, a compound of general formula (II) or a protected or activated derivative thereof is combined with a compound of general formula (III) in the presence of a su...
Embodiment 182
[0417] Prepared according to Example 182 from tert-butyl 4-[methyl(2-nitrobenzenesulfonyl)amino]piperidine-1-carboxylate (Preparation 74). δ H (d 6 -DMSO): 8.44 (2H, m), 8.13 (2H, m), 4.20-7.07 (1H, m), 3.29-3.17 (2H, m), 3.03-2.90 (2H, m), 2.77 (3H, s ), 2.00-1.84 (2H, m), 1.51-1.43 (2H, m).
[0418] Preparation 76: 5-Chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylic acid (1-(S)-(4-fluorobenzyl)-2-{4-[methyl(2-nitrate phenylsulfonyl)amino]piperidin-1-yl}-2-oxoethyl)amide
[0419]
Embodiment 231
[0420] From 2-(S)-[(5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carbonyl)amino]-3-(4-fluorophenyl)propanoic acid (Example 231) Example 230) and N-methyl-2-nitro-N-piperidin-4-ylbenzenesulfonamide hydrochloride (Preparation 75). m / z(ES + )=643.36[M+H] + .
[0421] Preparation 77: Thiomorpholine 1,1-dioxide
[0422]
[0423] To a solution of thiomorpholine (1.0 g, 9.69 mmol) in acetic acid (11.5 mL) cooled to 0 °C (ice bath) was added aqueous hydrogen peroxide (30% w / v, 4 mL) and the reaction was heated to 100 ℃ 16h. The mixture was cooled and the solvent was removed in vacuo, then the residue was triturated with methanol to give a white precipitate. The solid was filtered and washed with methanol to afford the title compound as an off-white powder. m / z(ES + )=136.06[M+H] + .
[0424] Preparation 78: Methyl piperidine-4-carboxylate hydrochloride
[0425]
[0426] To a cooled solution of anhydrous methanol (100 mL) was added acetyl chloride (7.1 mL, 0.1 mol) and the solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com