A method of producing titanium
A technology of titanium metal and ilmenite, applied in chemical instruments and methods, titanium compounds, inorganic chemistry, etc., can solve the problems of expensive titanium metal, high loss, and expensive precursors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
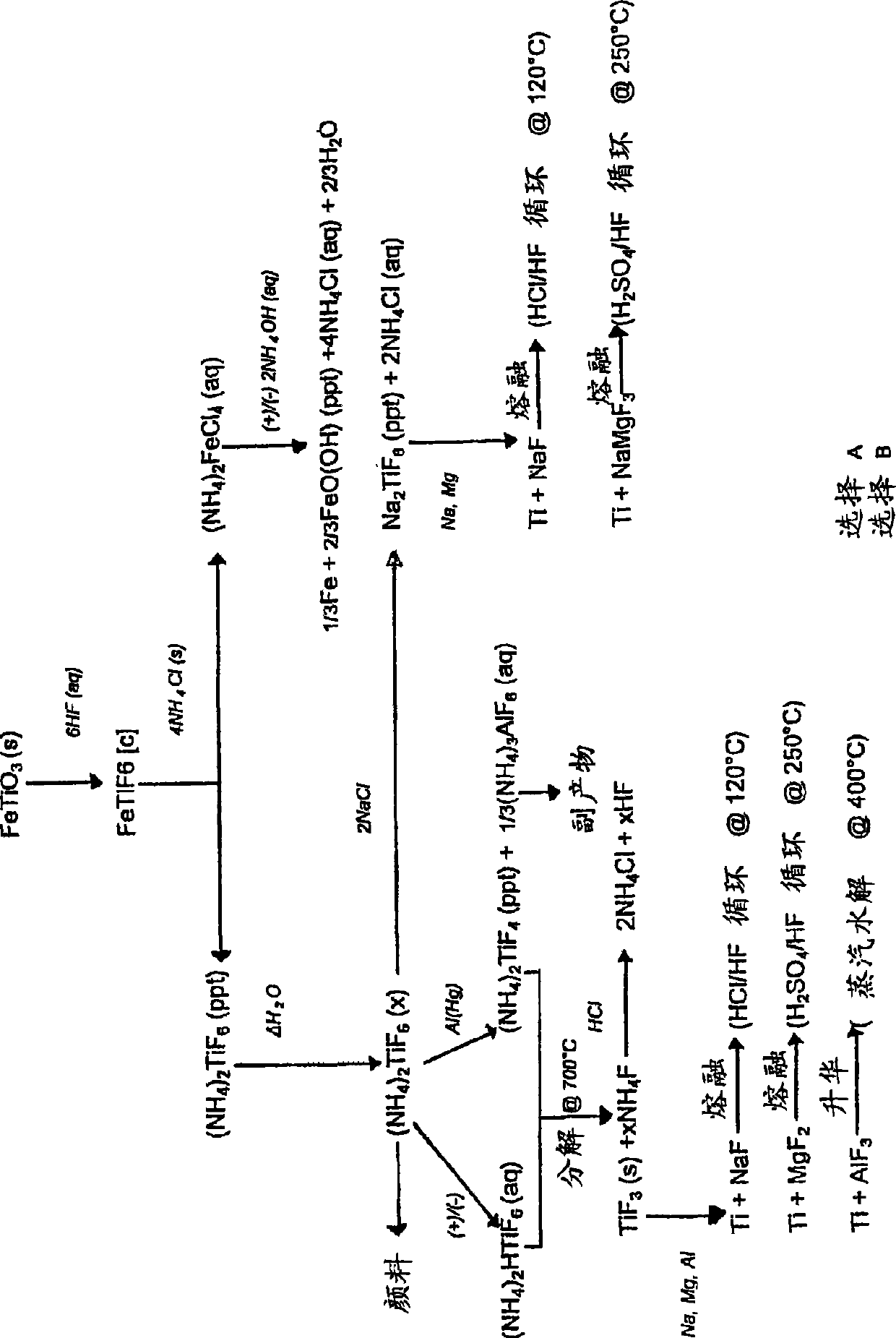
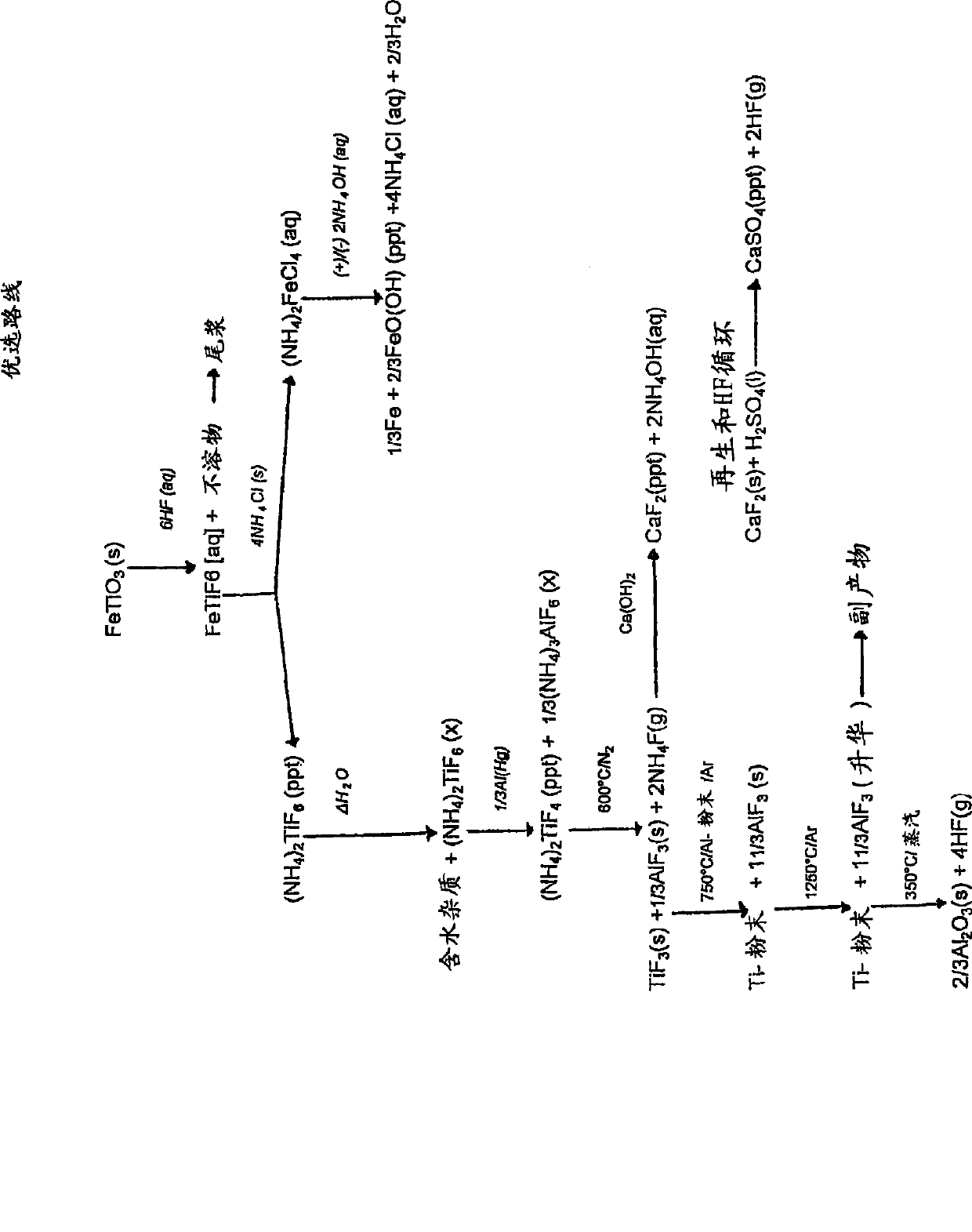
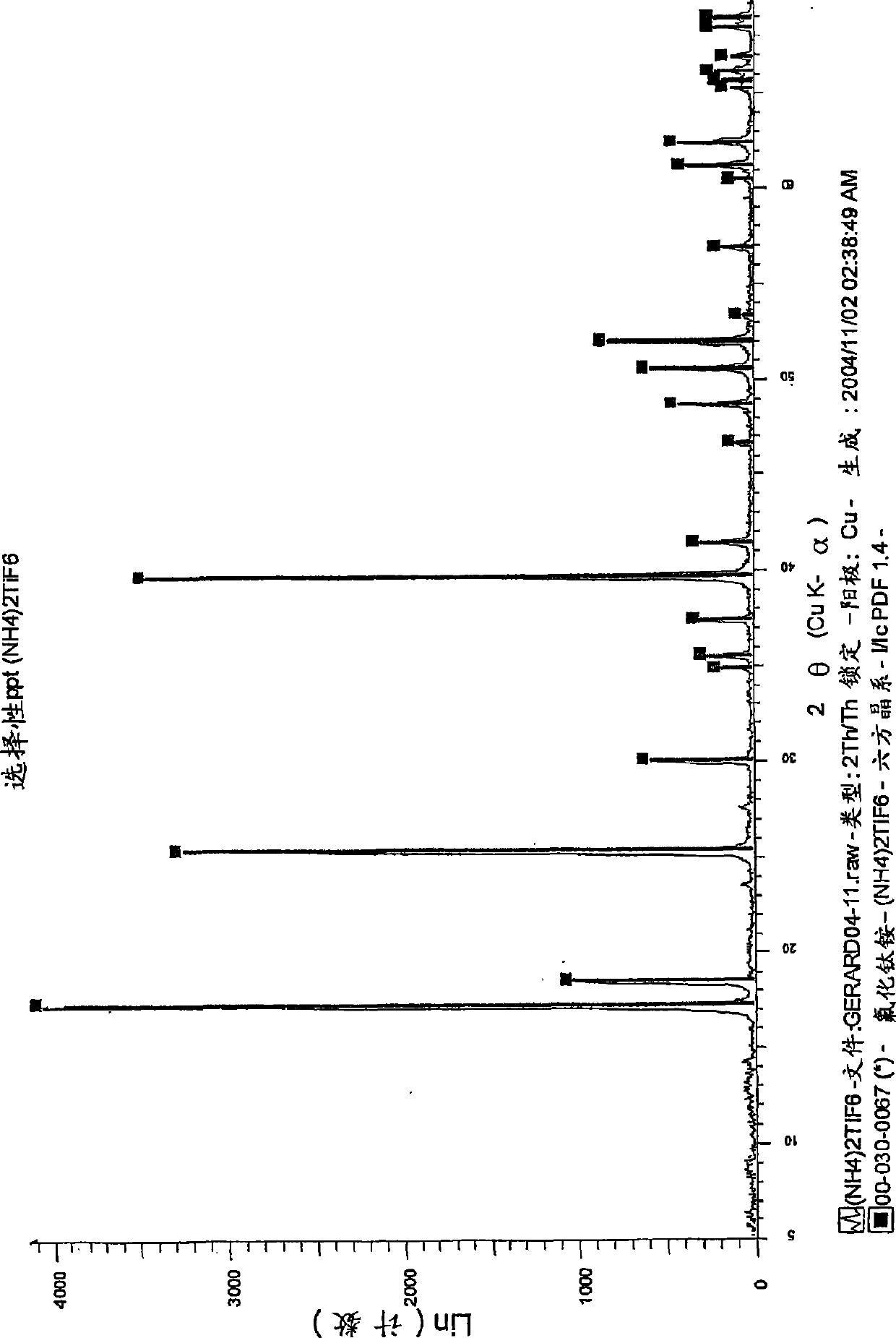
[0149] Reduction of (NH by Al(Hg) 4 ) 2 TiF 6 Titanium from ilmenite
[0150] Step 1: Digest ilmenite with diluted HF
[0151] Feed
[0152] Ilmenite concentrate is used as feed for the digestion step. The material contains approximately 89.5% ilmenite, 6% hematite, 2.5% quartz, and 2% other metal oxides. The particle size was uniform with about 98% of the material having a particle size between +45 μm and -106 μm. The material typically has the following chemical composition:
[0153] Al Ca Fe Mg mn Si Ti V 0.35% 0.1% 37.2% 0.27% 0.95% 1.18% 28.3% 0.5%
[0154] Stoichiometric: HF required for 500g ilmenite feed
[0155]The ilmenite used was composed of FeTiO 3 (89.5%), Fe 2 o 3 (6.0%), SiO 2 (2.5%) and other substances (2%). This is equivalent to counting as 500g of FeTiO 3 (447.5g; 2.95mol), Fe 2 o 3 (30g; 0.19mol) and SiO 2 (12.5 g; 0.21 mol). per mole of FeTiO 3 , Fe 2 o 3 and SiO 2 Each requires 6 mol...
Embodiment 2
[0214] Preparation of Titanium-Vanadium Alloy
[0215] Step 1: Preparation of NH 4 VF 4 and VF 3
[0216] To manufacture titanium alloys, such as Ti-6Al-4V, alloying elements in the form of metal fluorides are combined with TiF in appropriate proportions before reduction with Al 3 mix. In the case of Ti-6Al-4V, VF 3 was added to TiF 3 , and use 6% excess Al in the reduction process to make the AlF 3 Alloy powder is produced after sublimation.
[0217] V cannot be VF 5 or VF 4 Introduced because these compounds have low boiling points and they may undergo sublimation prior to reduction. Therefore, VF must be prepared as follows 3 As a V precursor.
[0218] Will NH 4 VO 3 (58.5g) was added to water (300ml) and stirred. Will NH 4 Cl (53.5 g) and HF (40%; 130 ml) were added to the resulting solution resulting in a yellow solution.
[0219] Fe (14 g, steel wire) was added to the solution to reduce V(V) to V(IV). The reaction was exothermic and a blue solutio...
Embodiment 3
[0227] By (NH 4 ) 2 FeCl 4 Solution regeneration NH 4 Cl
[0228] If using NH 4 OH as a by-product (NH 4 ) 2 Precipitation of Fe(OH) in FeCl4 solution 2 , the resulting problem is that at high concentrations of NH 4 Solubility in Cl. This leads to very slow precipitation. Moreover, Fe(OH) 2 Oxidized by air to FeO(OH) (in NH 4 low solubility in Cl) is slower and impractical, use H 2 o 2 Oxidation is more effective but the reagent is expensive.
[0229] The applicant has found that the oxidation of Fe(II) to Fe(III) can be enhanced by introducing an electric current into the solution. The following reactions occur:
[0230] (NH 4 ) 2 FeCl 4 +Current=Fe+Cl 2 +2NH 4 Cl
[0231] Cl 2 +2(NH 4 ) 2 FeCl 4 =2FeCl 3 +4NH 4 Cl
[0232] 2FeCl 3 +6NH 4 OH=6NH 4 Cl+2FeO(OH)+2H 2 O
[0233] 3(NH 4 ) 2 FeCl 4 +6NH 4 OH + current = 12NH 4 Cl+Fe+2FeO(OH)+2H 2 o
[0234] Therefore, by adding NH while stirring 4 OH, 1 liter (NH 4 ) 2 FeC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com