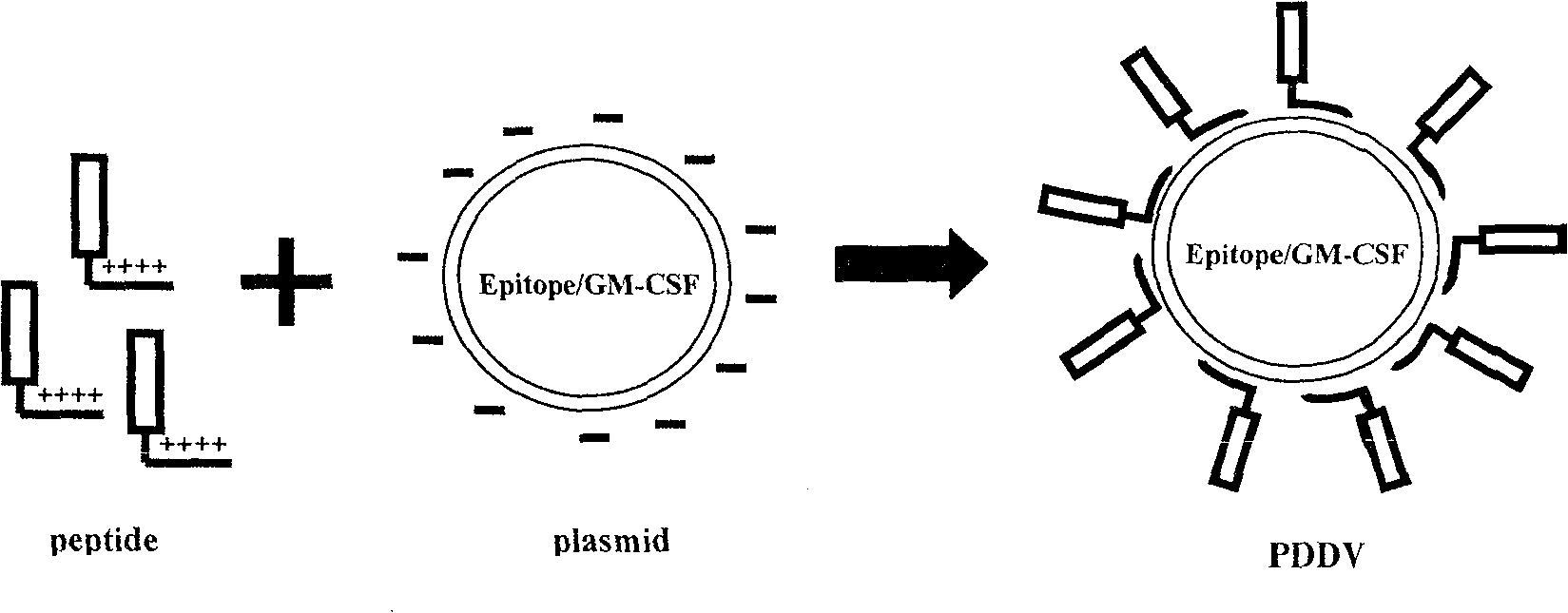
Peptide-DNA double vaccine based on T-cell epitope for anti-Schistosoma japonicum infection
A technology of epitope peptide and schistosomiasis, applied in the field of immunology, can solve the problem of unclear T epitope and achieve the effect of reducing cross-reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of PDDV
[0034] Using various software such as SYFPEITHI and TEPITOPE to predict and analyze the T cell epitope of the full-length antigen of Schistosoma japonicum 22.6kDa surface membrane protein, and then select the epitope with the highest score after homology comparison and restriction of restriction enzyme sites. position, its amino acid sequence is AKQYNICCKFKELLD (Ala Lys Gln Tyr Asn Ile Cys Cys Lys Phe Lys Glu Leu Leu Asp).
[0035] Build process like figure 1 as shown,
[0036] 1) Order the peptide of the above sequence from Invitrogen and add 18 lysines KKKKKKKKKKKKKKKKKKAKQYNICCKFKELLD (purity ≥ 99%) to its amino terminus during synthesis, so that the entire synthetic peptide is positively charged and named P5. In addition, to set up a control group, we also ordered a peptide segment (purity ≥ 99%) containing only 18 lysines KKKKKKKKKKKKKKKKKK, and named it 18K.
[0037] 2) Add a Sal I restriction site to the 5' end of the epitope p...
Embodiment 2
[0061] Embodiment two: carry out immunity / challenge infection experiment with the PDDV vaccine that embodiment one makes
[0062] Clean grade C57BL / 6 mice: purchased from the National Mouse Genetic Resources Celebration Center of Nanjing University, 8 weeks old, female.
[0063] First experiment:
[0064] A) The mice were randomly divided into three groups, 14 in each group, named as T5-PDDV group, 18K-PDDV group and control group respectively.
[0065] B) Immunize once a week, subcutaneously inject 100 μl of T5-PDDV solution (containing 28 μg P5 peptide segment and 5 μg recombinant eukaryotic expression plasmid DNA) and 18K-PDDV solution (containing 28 μg 18K peptide segment and 5 μg empty eukaryotic expression plasmid DNA) or PBS solution.
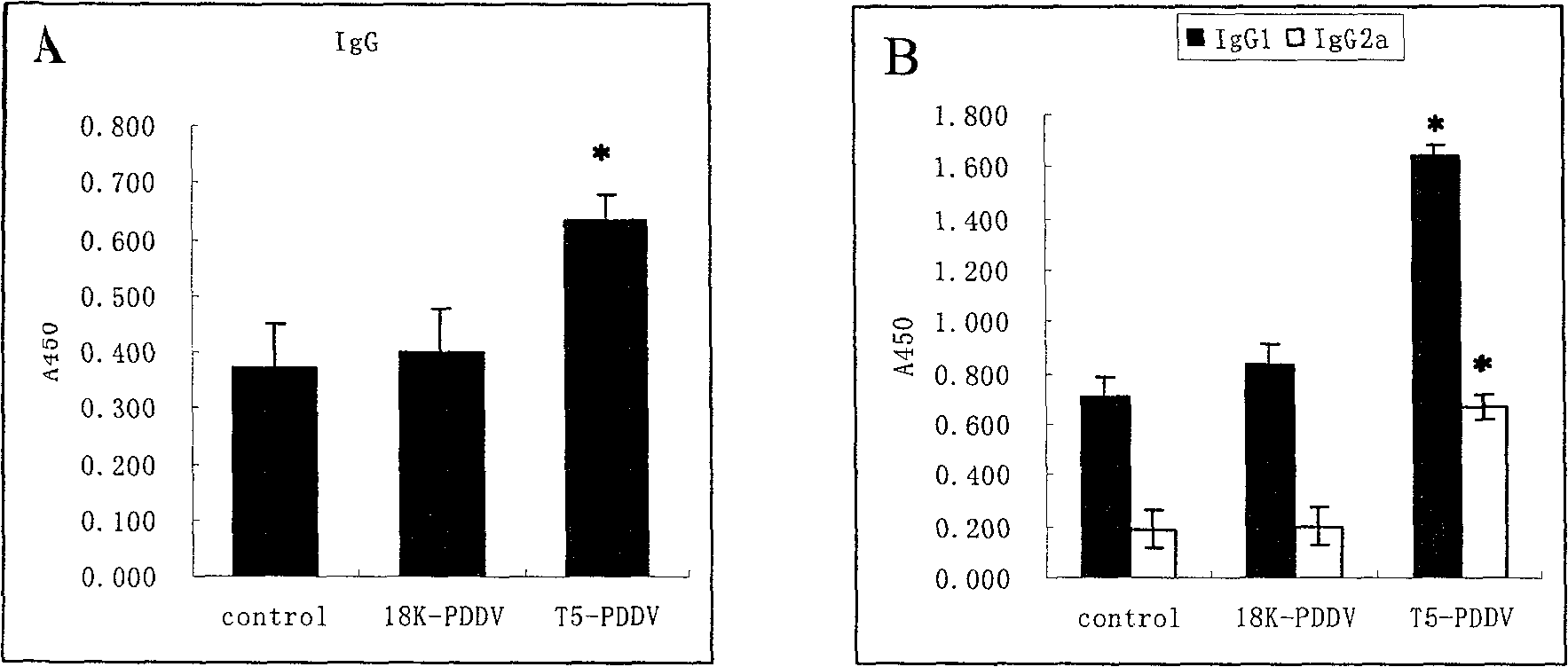
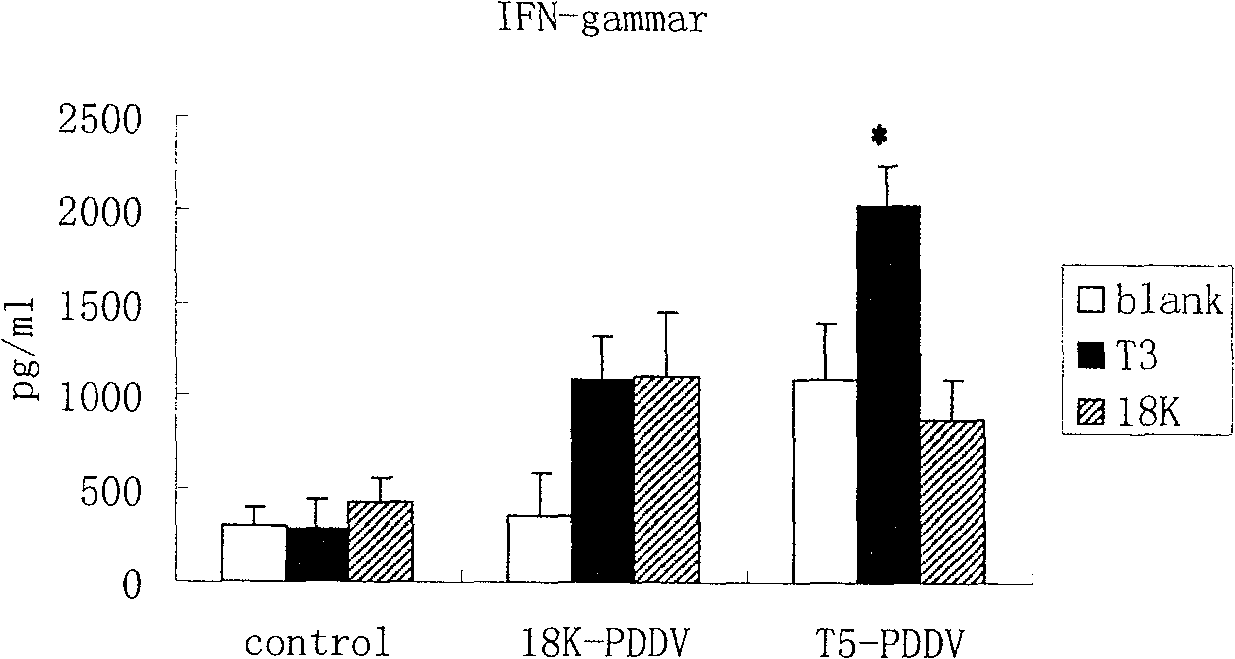
[0066] C) Detection of the immune response induced by PDDV vaccine in mice: ① before immunization and 2 weeks after the last immunization, blood samples were taken from the tail vein of the mice, and used for ELISA to detect the seru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com