Kutkin derivative, preparation method and application thereof
A technology of berberine and derivatives, applied in the field of berberine derivatives and preparation thereof, can solve problems such as side effects in the gastrointestinal tract, and achieve the effects of no toxic and side effects, low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
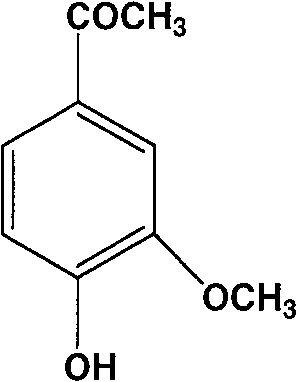
[0026] The synthesis of embodiment 14-acetoxy-3-methoxybenzophenone
[0027] Acetyl chloride (1.28ml, 18mmol) was added dropwise to berberine (2g, 12mmol) 20ml ethyl acetate solution cooled to 0°C, and triethylamine was added dropwise under nitrogen, and the reaction solution was gradually warming up to room temperature and stirred for 2 hours. The reactant was washed 3 times with water (50mlx3), the organic layer was dried with anhydrous sodium sulfate, and recrystallized with ethyl acetate to obtain 1.8g of white crystals with a yield of 72%. mp 56-57°C. 1 H NMR (acetone-d 6 , ppm): 7.67-7.56 (m, 2H, Ar-H), 7.21-7.13 (d, 1H, Ar-H), 3.88 (s, 3H, OCH 3 ), 2.57 (s, 3H, COCH 3 ), 2.27 (s, 3H, O COCH 3 ), the molecular formula of MS 208. product 4-acetoxy-3-methoxybenzophenone is as follows:
[0028]
Embodiment 24
[0029] The synthesis of embodiment 24-hydroxyl-3-methoxyl group-5-nitrobenzophenone
[0030] In berberine (10.2g, 61mmol) 500ml chloroform solution, concentrated nitric acid (70%, 16.3ml) was added dropwise at 0°C, the reaction solution was kept stirring for 2 hours, the reaction solution was washed 7 times with water (70mlx7), and the organic layer was washed with water for 7 times (70mlx7). Dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, add 250ml of 95% ethanol, and crystallize overnight to obtain 9.5g of a yellow needle-like product with a yield of 73%. mp 159-161℃. 1 H NMR (CDCl 3 , ppm): 11.13 (s, 1H, OH), 8.32-8.31 (d, 1H, Ar-H), 7.78-7.77 (d, 1H, Ar-H), 4.02 (s, 3H, OCH 3 ), 2.64 (s, 3H, COCH 3 ), MS 211.
[0031] The molecular formula of product 4-hydroxyl-3-methoxyl group-5-nitrobenzophenone is as follows:
[0032]
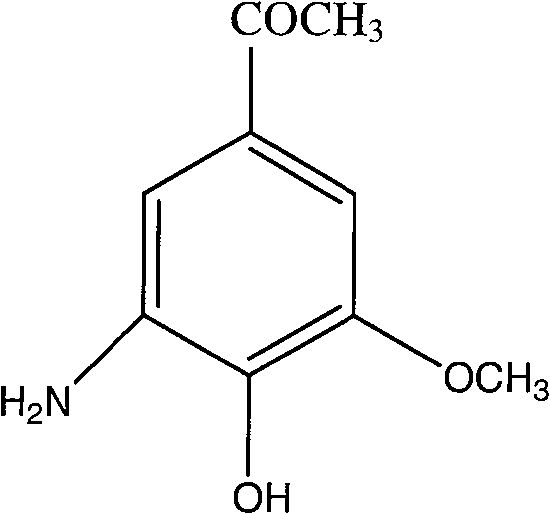
Embodiment 35
[0033] Synthesis of Example 35-amino-4-hydroxyl-3-methoxy-benzophenone
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com