Chinese medicine composition for treating endotoxemia and its preparation process
A technology of anti-endotoxin and composition, which is applied in the direction of drug combination, pharmaceutical formula, antibacterial drug, etc., can solve the problems of reducing mortality, difficulty in regulating and blocking systemic inflammatory response, unrealistic multiple monoclonal antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, in vitro endotoxin experiment
[0023] 1.1 Experimental materials
[0024] All Chinese herbal medicines used have been identified as genuine products; freeze-dried Escherichia coli endotoxin standard (Beijing Institute of Biological Products Inspection); Limulus reagent (TAL) (Zhanjiang Andus Biotechnology Company); Dusi Biological Co., Ltd.); endotoxin quantitative assay kit (Shanghai Yihua Biological Products Co., Ltd.); endotoxin (Sigma); polymyxin B (Sigma); transmission electron microscope CH-600 Hitachi).
[0025] 1.2 method;
[0026] 1.2.1 preparation preparation
[0027] The anti-endotoxemia pharmaceutical composition by weight: capillary 20%, gardenia 20%, rhubarb 10%, salvia miltiorrhiza 9%, honeysuckle 20%, forsythia 9%, sunburned astragalus 9%, licorice or roasted licorice 3 % Combined deployment, except for rhubarb, decoct 3 times, 20 minutes each time, combine the decoction to 1 / 2-1 / 6 volume, add rhubarb and decoct the decoction for 40-50 ...
Embodiment 2
[0033] Embodiment 2, in vivo anti-endotoxin activity experiment
experiment approach I
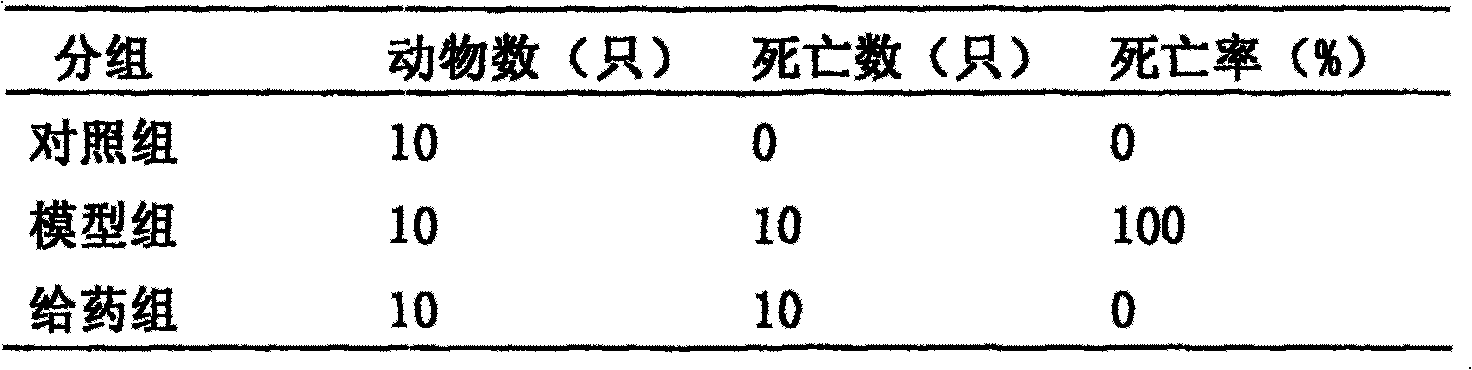
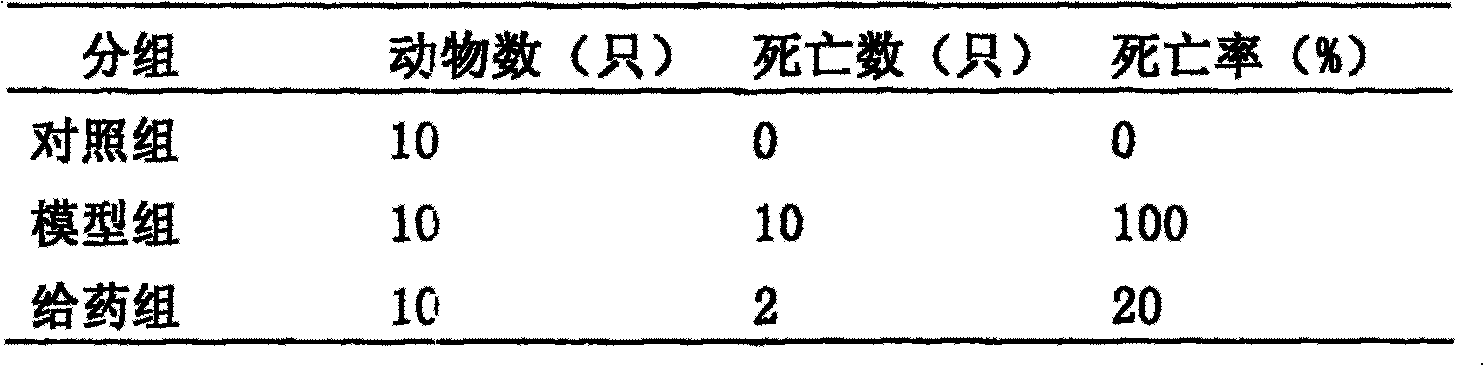
[0034] 2.1 Experimental scheme I: Modeling with endotoxin + lead acetate (PbAC)
[0035] 2.1.1 Experimental materials
[0036] The anti-endotoxemia pharmaceutical composition by weight: capillary 20%, gardenia 20%, rhubarb 10%, salvia miltiorrhiza 9%, honeysuckle 20%, forsythia 9%, sunburned astragalus 9%, licorice or roasted licorice 3 % Combined deployment, except for rhubarb, decoct 3 times, 20 minutes each time, add rhubarb and decoct for 40-50 minutes, combine the decoction to contain 1 gram of crude drug per milliliter. Endotoxin (LPS Sigma Company), lead acetate (PbAC analytically pure), Kunming white mice, half male and half female, body weight (20±2) g, were purchased from the Experimental Animal Center of Hubei Province.
[0037] 2.1.2 Experimental methods and results
[0038] Accurately weigh the endotoxin and make a 0.4 mg / ml solution with saline. Accurately weigh PbAC and make a 4 mg / ml solution with double distilled water. 30 mice were divided into 3 groups, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com