Fusion protein in soluble receptor II - antibody Fc section of human tumor necrosis factor
A tumor necrosis factor and fusion protein technology, applied in the field of human tumor necrosis factor soluble receptor II-antibody Fc segment fusion protein, can solve the problems of large side effects and insignificant effects of drugs, and achieve the goal of improving expression level and reducing screening intensity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 PCR amplification gene: with SEQ ID No.4 (5'-AAAAAA GTTTAAAC ATGGTTCGACCATAGAACT-3') is the forward primer, with SEQ ID No.5 (5'AAAAAA GCTAGC TTAGTCTTTTTCCCTCGTAGACT-3') was used as the reverse primer, and the CHO cell cDNA library (Clontech Company) was used as the template. The PCR reaction was carried out in a total volume of 50 μL, and the reaction conditions were denaturation at 94°C for 5 minutes, followed by cycling at 94°C for 50 seconds. , annealing at 58°C for 1.5 minutes, extension at 72°C for 1 minute, a total of 30 cycles, and then extending at 72°C for 10 minutes, amplified a DNA fragment SEQ ID No.3 consistent with the expected size (564bp).
Embodiment 2
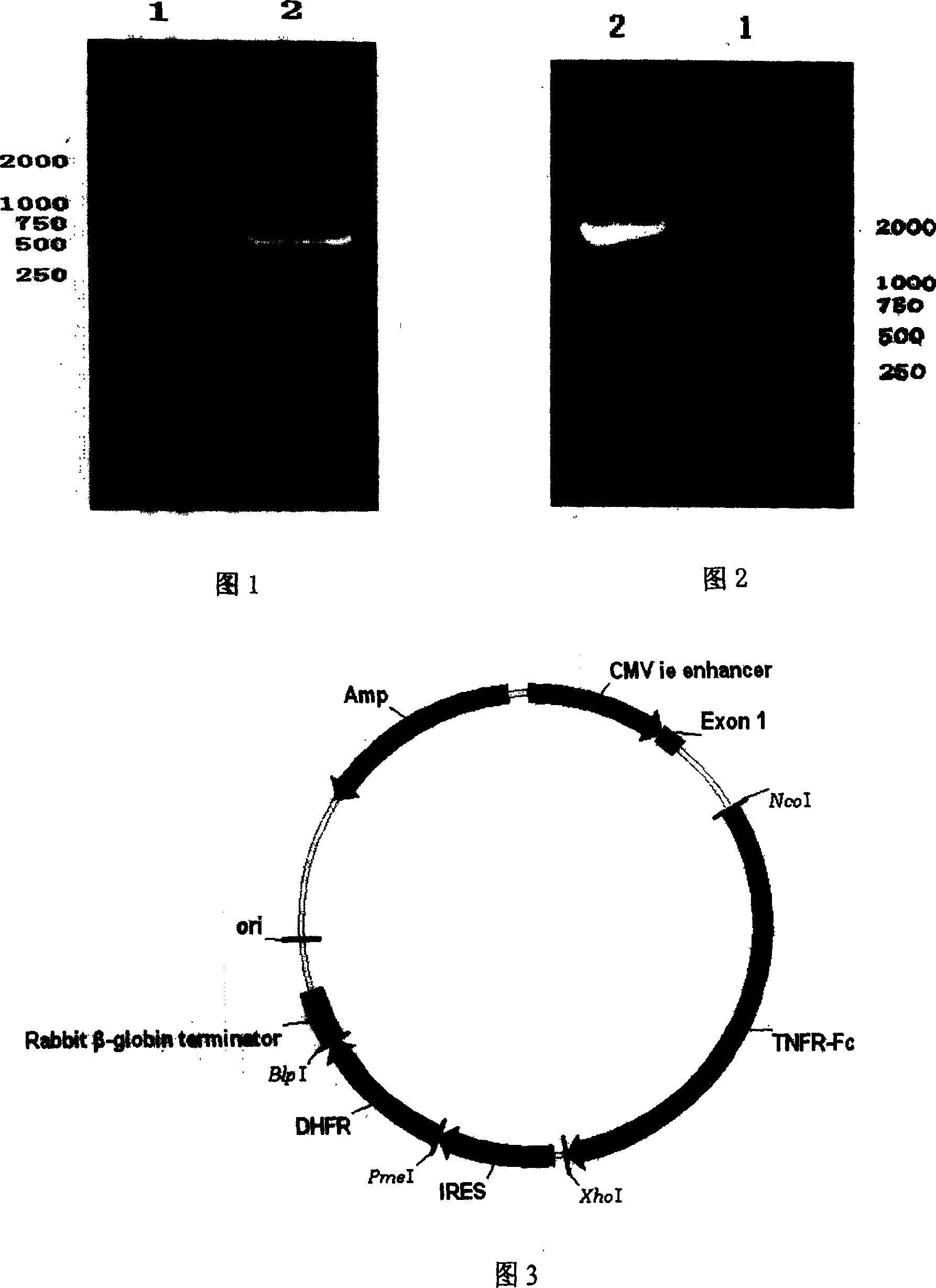
[0016] Example 2 Construction of pT-dhfr expression vector: The gel-recovered PCR product and the vector pTandem-1 (Novagen) were double-digested with PmeI and BlpI, respectively, and the reagents were recovered from 1% agarose gel by gel The box was recovered, then ligated (16°C, 16h), transformed into Escherichia coli DH-5α competent cells, positive clones were selected, plasmids were extracted and verified by double enzyme digestion analysis, and DNA sequencing was carried out for identification. The sequencing results were consistent with SEQ ID No. 3. The sequences are identical, and the constructed expression plasmid is called pT-dhfr. The construction strategy is shown in FIG. 3 .
Embodiment 3
[0017] Example 3 Two primers of SEQ ID No.8 and SEQ ID No.9 were synthesized according to SEQ ID No.6, and the human lung cDNA library (Clontech Company) was used as a template for PCR reaction to amplify human tumor necrosis factor soluble receptor II gene sequence (TNFRII), the PCR product is TNFRII-pcrp.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com