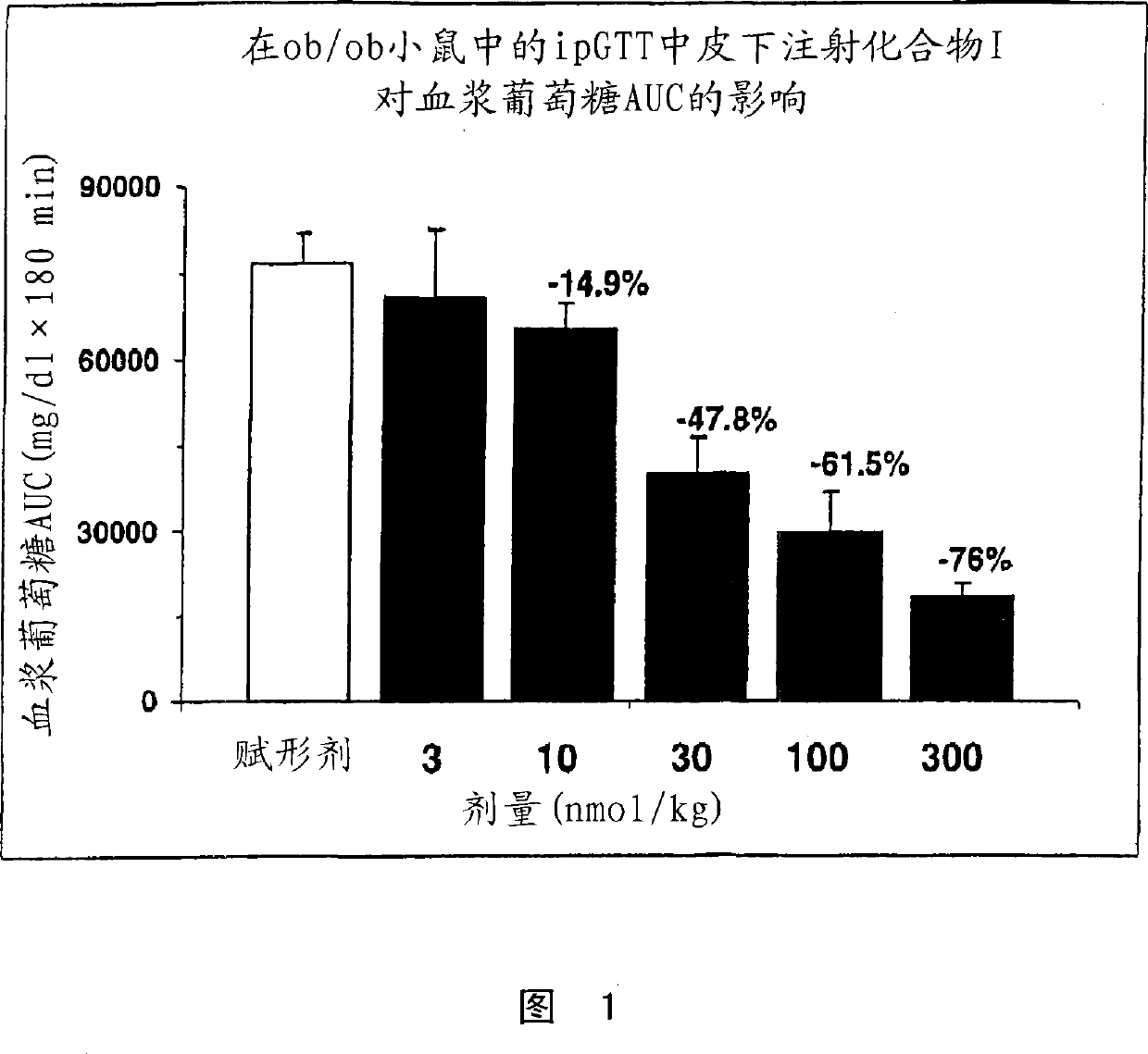
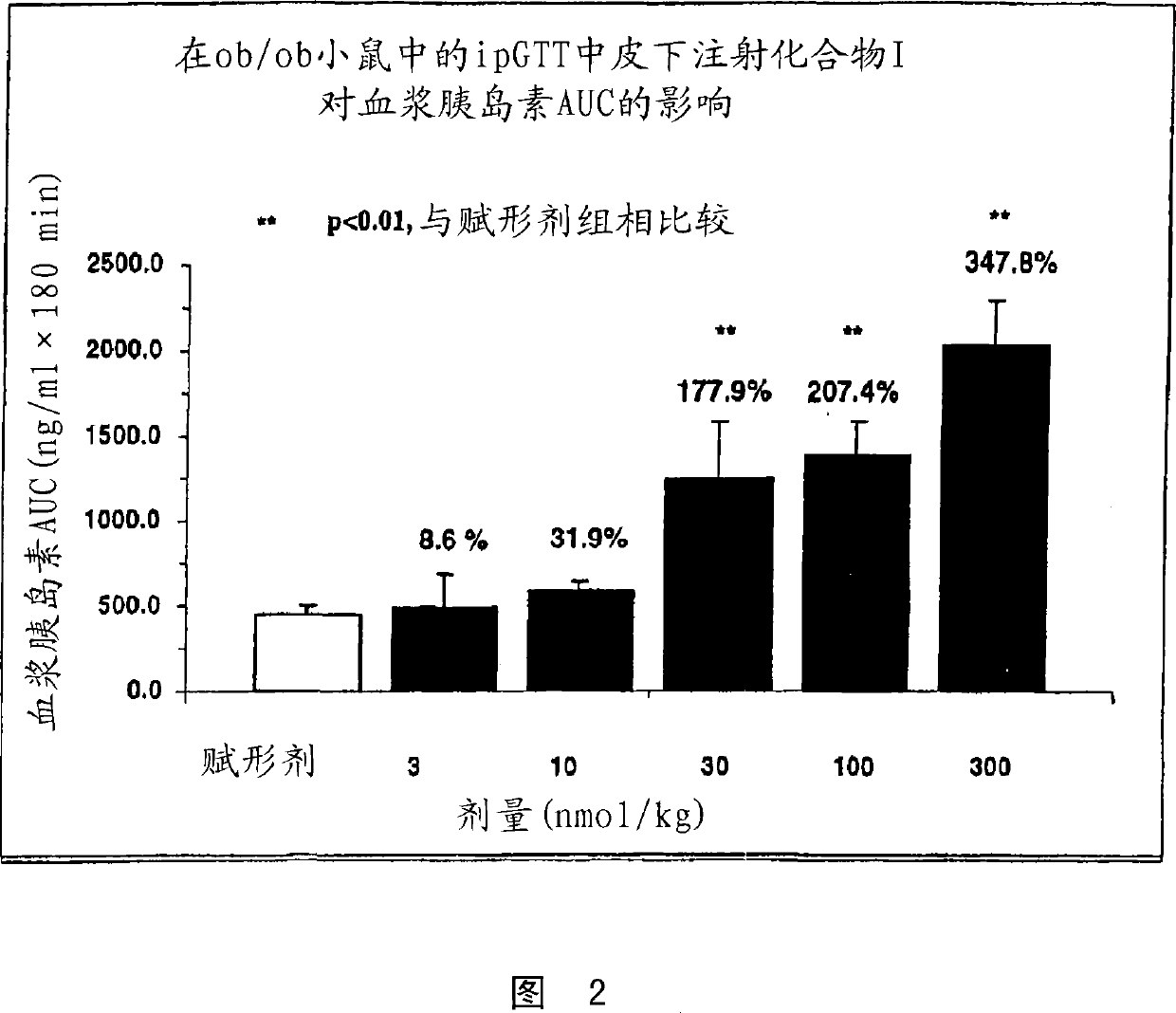
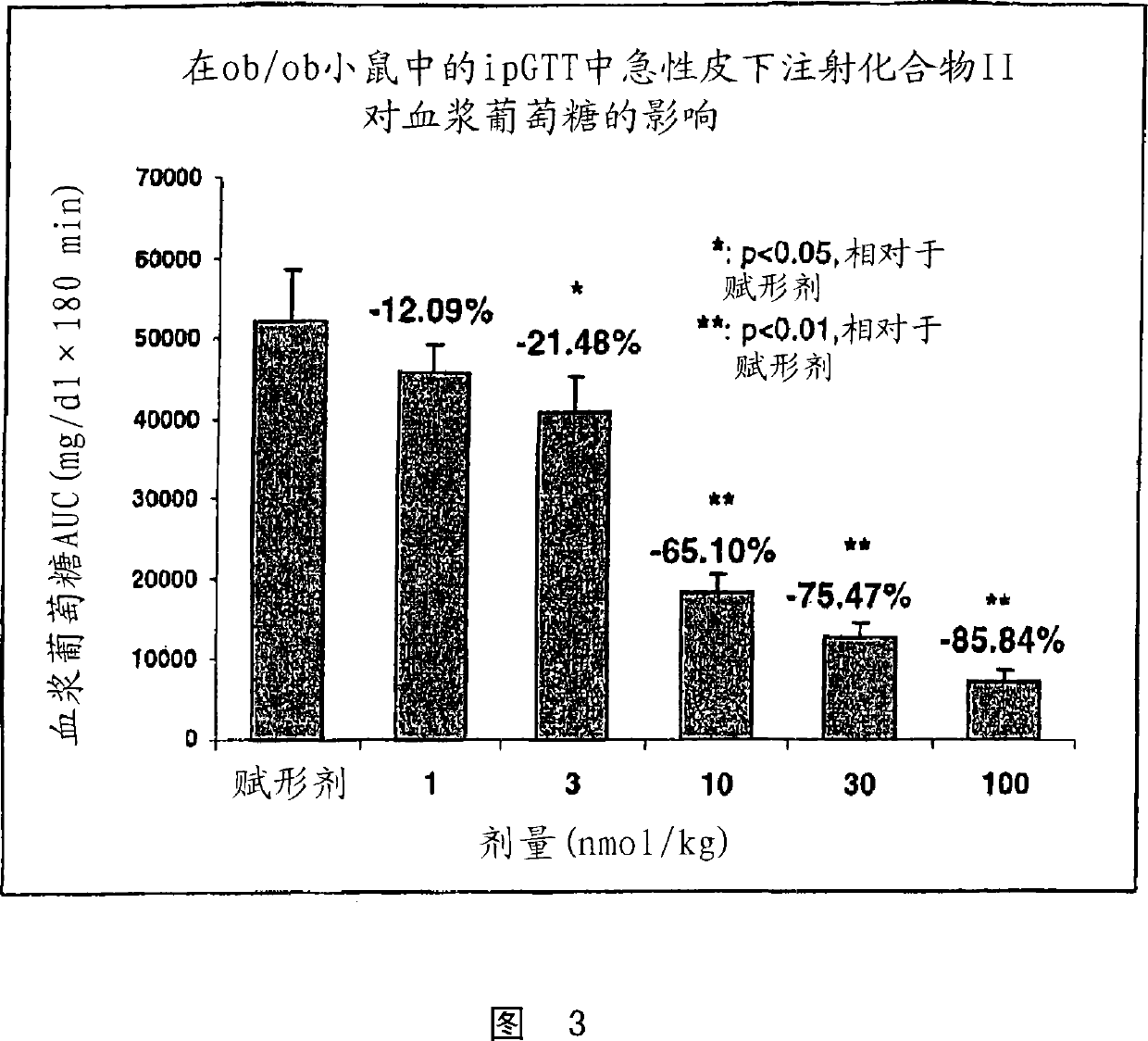
Human glucagon-like-peptide-1 modulators and their use in treatment of diabetes and related conditions
A halogen and amino acid technology, applied in the direction of glucagon, hormone peptide, animal/human protein, etc., can solve the problem of short serum half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0366] Simultaneous solid phase peptide synthesis of 11-segment peptide
[0367] Prepare in position X in batches using the following manual procedures aa10 And X aa11 The dipeptidyl resin containing amino acids is then used on the MPS-396 peptide synthesizer to perform continuous peptide chain extension using automated simultaneous synthesis procedures. The synthesis of N-α-Fmoc-protected biphenylalanine or phenyl-heteroaryl-alanine derivatives used in the manual coupling is described in the above general experimental method, and as Described in Examples 10-16 and 21-22.
[0368] 9-Fmoc-aminoxanthene-3-yloxy-Merrifield resin (Sieber amide resin) in an amount sufficient to synthesize several 11-link analogs was washed with DMF (4×10 mL / g, 5 minutes); Load: 0.5 to 0.7mmol / g) swelling. Then, it was treated twice with 20% piperidine in DMF solution (10 mL / g) for 5 and 15 minutes, respectively, to remove the Fmoc group. The resin was washed with DMF (4×10 mL / g) and NMP (4×10 mL / g). P...
Embodiment 2
[0393] A. General method for the synthesis of N-acylated 11-segment peptide analogs (Scheme 2).
[0394] As shown in Flow Diagram 2, the synthesis of the N-acylated 11-segment peptide analogue is from the protected 11-segment peptidyl-resin intermediate prepared by the method described herein (1) (0.015 mmol) start. Use the method described here to remove the Fmoc group, and use the coupling process described in the general method described here to couple the resulting resin intermediate 2 with the relevant Fmoc protected amino acid or carboxylic acid . In the case where a suitable anhydride is available, the N-acylation is performed using 5 equivalents of anhydride in NMP solution. The resulting N-acylated 11-link analog (3) was cleaved / deprotected according to the general method described herein and purified by preparative HPLC.
[0395] Scheme 2: Synthesis of 11-linked peptide analogs substituted / derivatized with residue #1
[0396]
[0397] B. General formula for the synt...
Embodiment 3
[0406] Use Applied Biosystems Model 433A Peptide Synthesizer for 11-segment peptides Solid phase synthesis of analogs
[0407] The following is a general description of the solid-phase synthesis of typical 11-segment peptide analogs using the upgraded Applied Biosystems Model 433A peptide synthesizer. The upgraded hardware and software of the synthesizer can conduct conductive monitoring of the Fmoc deprotection step along with the coupled feedback control. The procedure allows for synthesis scales in the range of 0.05 to 1.0 mmol.
[0408] The incorporation of the two unnatural C-terminal amino acids related to the simultaneous synthesis of 11-link analogs is as described above. This Fmoc-protected dipeptidyl resin was used in the synthesis of this ABI. The Fmoc-protected dipeptidyl-resin (0.1 mmol) was placed in an appropriately sized container on the instrument, washed 6 times with NMP and treated twice with 22% piperidine / NMP ( Each 2 and 8min.) to deprotect. One or two addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com