Modified glucagon sample peptide-1analogue and modifying matter, and uses thereof
A technology for glucagon and analogs, applied in the field of novel glucagon-like peptide-1 analogs and their modifications, which can solve the problems of long pharmacological action time, lack of hypoglycemic effect and stability , to achieve stability and increase in vivo action time, superior biological characteristics, and the effect of promoting insulin secretion and lowering blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Chemical synthesis of glucagon-like peptide-1 analogues
[0064] The analogs of the present invention can be prepared by conventional solid-phase peptide synthesis methods to prepare the GLP-1 analogs involved in the present invention, and the specific operation is entrusted to Shanghai Jier Biochemical Company to complete.
[0065] One of the polypeptides of the present invention is synthesized by solid-phase synthesis, and the sequence is:
[0066] SEQ NO 1: KHAEGTFTSDVSSYLEGQAAKEFIAWLVKGR-NH 2
[0067] Other peptide synthesis methods are similar.
[0068] The synthesis process adopts Fmoc synthesis method, and Rink-Amide-MBHA Resin is selected for synthesis. The synthesis steps are as follows:
[0069] (1) 16 kinds of Fmoc-amino acid raw materials with side chain protecting groups
[0070] (2) Solid phase synthesis
[0071] (3) Remove the side chain protecting group
[0072] (4) HPLC purification
[0073] (5) Freeze drying
[0074] (6) GLP-1 analogs.
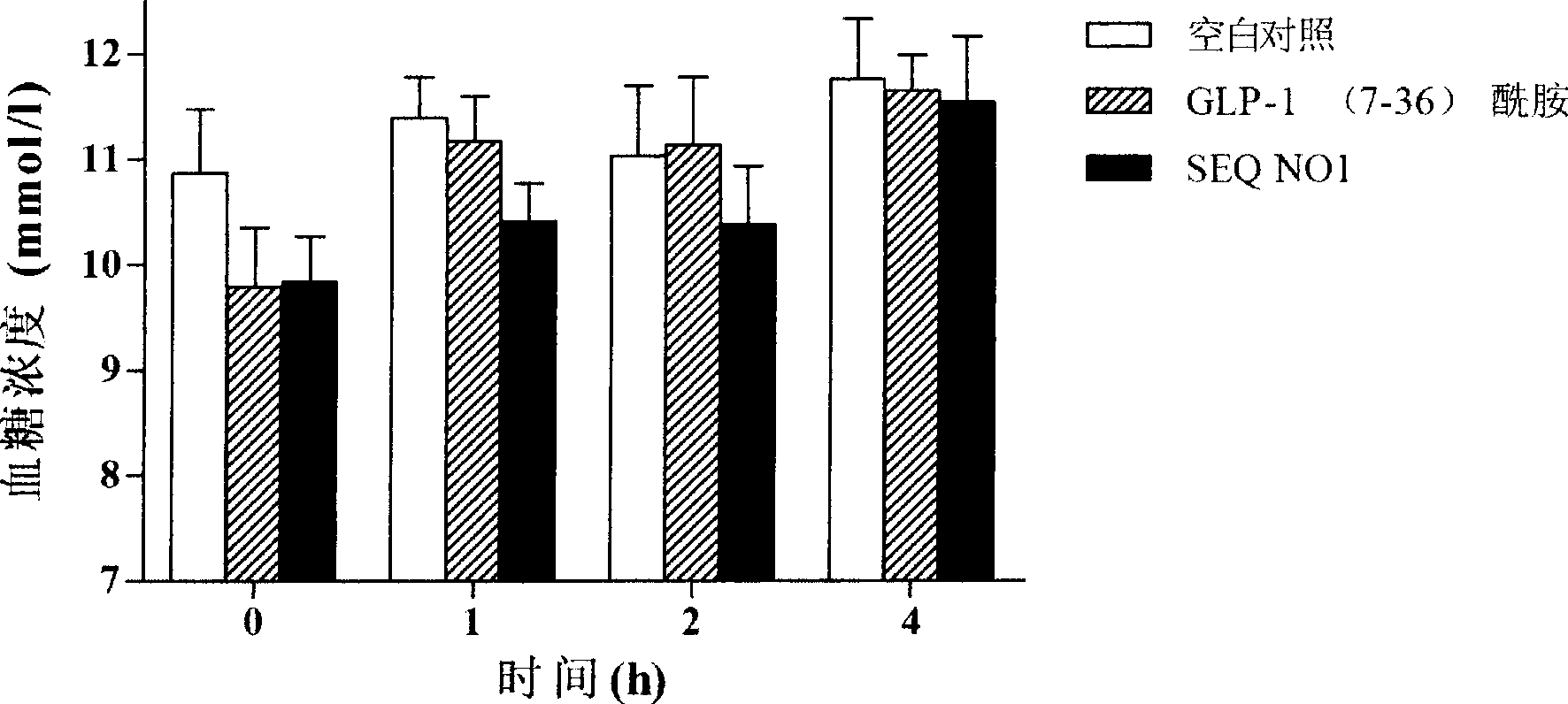
Embodiment 2
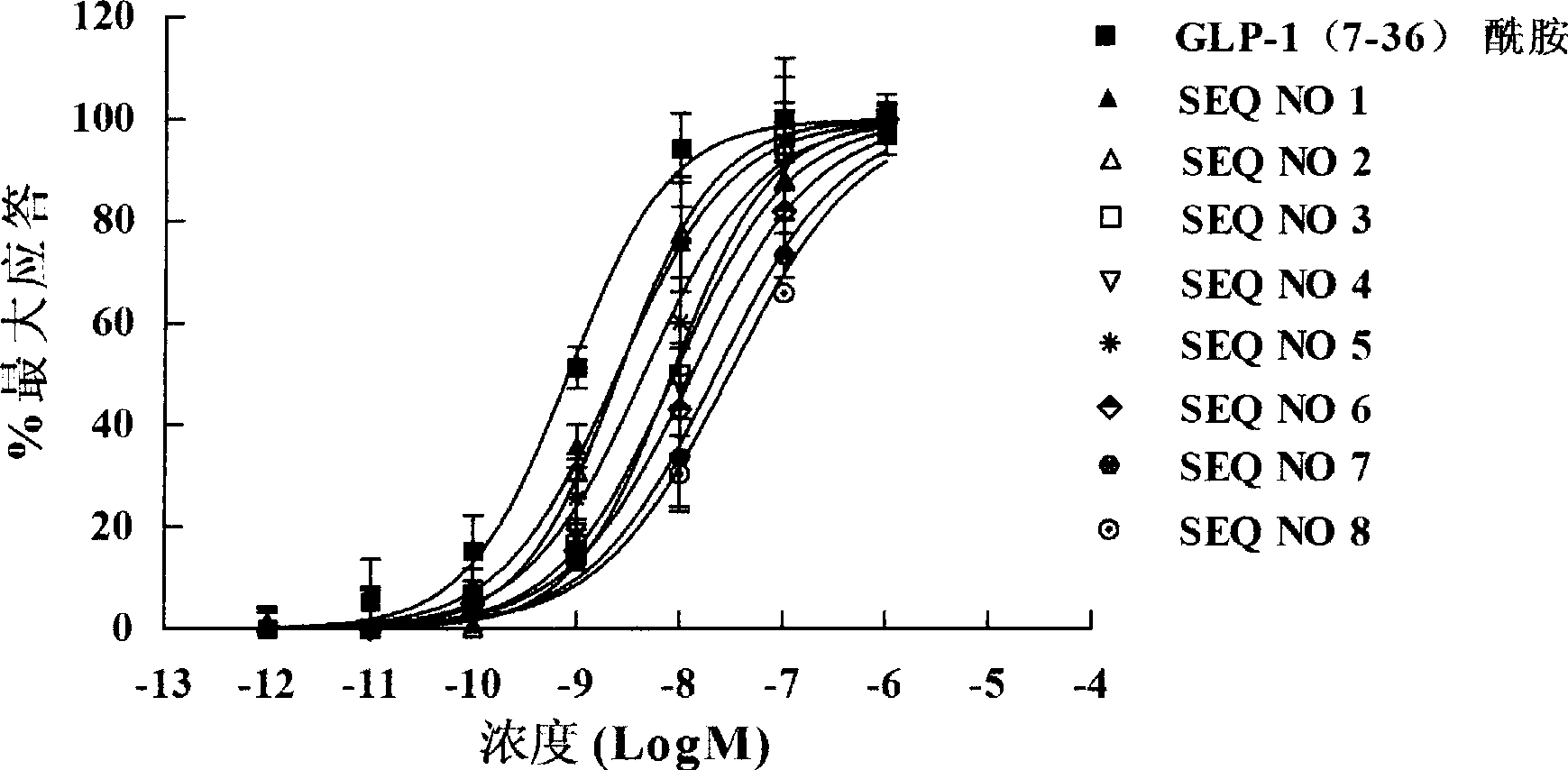
[0075] Example 2 In vitro activity determination of GLP-1 analogs
[0076] Test sample:
[0077] SEQ NO 1: KHAEGTFTSDVSSYLEGQAAKEFIAWLVKGR-NH 2
[0078] SEQ NO 2: KHAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG
[0079] SEQ NO 3: KHAEGTFTSD SSYLEGQAAKEFIAWLVKGR-NH 2
[0080] SEQ NO 4: KHAEGTFTSDV SYLEGQAAKEFIAWLVKGR-NH 2
[0081] SEQ NO 5: KHAEGTFTSDVSS LEGQAAKEFIAWLVKGR-NH 2
[0082] SEQ NO 6: KHAEGTFTSDVSSYLEGQAAK FIAWLVKGR-NH 2
[0083] SEQ NO 7: KHAEGTFTSDVSSYLEGQAAKEF AWLVKGR-NH 2
[0084] SEQ NO 8: KHAEGTFTSDVSSYLEGQAAKEFIA LVKGR-NH 2
[0085]GLP-1(7-36) amide is a positive control: HAEGTFTSDVSSYLEGQAAKEFIAWLVKGR-NH 2
[0086] experiment method:
[0087] Inoculate CHO / EGFP / GLP-1R cells in a 96-well plate with a density of 2×10 4 RPMI-1640 complete culture medium containing 100mg / L Zeocin was cultured for 48 hours and then the culture medium was removed, and the above-mentioned GLP-1 analogues (SEQ NO 1, SEQ NO. 1 and SEQ ID NO. 1) were serially diluted with RPMI-1640 basic me...
Embodiment 3
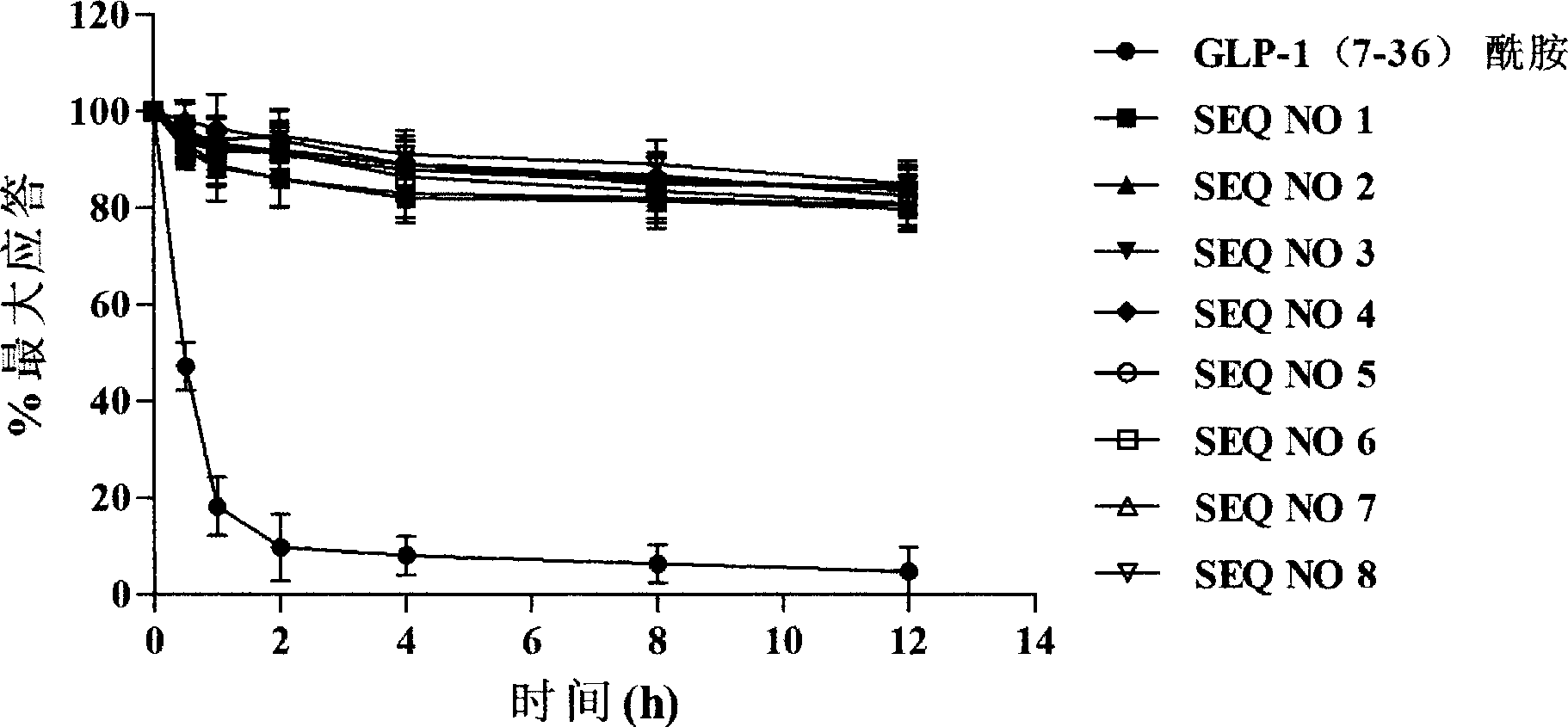
[0090] Example 3 In vitro anti-DPP-IV degradation determination of GLP-1 analogs
[0091] Test sample:
[0092] SEQ NO 1: KHAEGTFTSDVSSYLEGQAAKEFIAWLVKGR-NH 2
[0093] SEQ NO 2: KHAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG
[0094] SEQ NO 3: KHAEGTFTSD SSYLEGQAAKEFIAWLVKGR-NH 2
[0095] SEQ NO 4: KHAEGTFTSDV SYLEGQAAKEFIAWLVKGR-NH 2
[0096] SEQ NO 5: KHAEGTFTSDVSS LEGQAAKEFIAWLVKGR-NH 2
[0097] SEQ NO 6: KHAEGTFTSDVSSYLEGQAAK FX 29 AX 31 LVKGR
[0098] SEQ NO 7: KHAEGTFTSDVSSYLEGQAAKE AWLVKGR-NH 2
[0099] SEQ NO 8: KHAEGTFTSDVSSYLEGQAAKEFIA LVKGR-NH 2
[0100] GLP-1(7-36) amide is a positive control: HAEGTFTSDVSSYLEGQAAKEFIAWLVKGR-NH 2
[0101] experiment method:
[0102] Take 20 g of fresh pig kidney cortex tissue and chop it, add 0.25 mol / L sucrose solution at 20% (w / v), and homogenize with a homogenizer. The homogenate was centrifuged at 8,000 rpm for 15 min, and then the supernatant was centrifuged at 15,000 rpm for 2 hours. The precipitate was dissolved in 0.1 mol / L Tris / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com