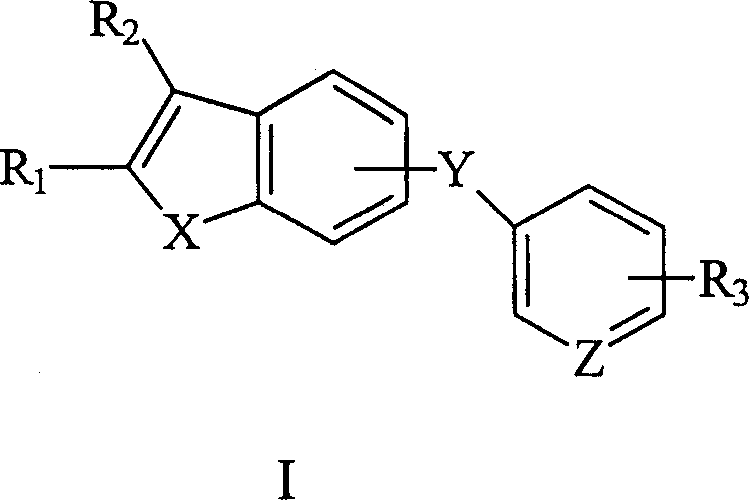
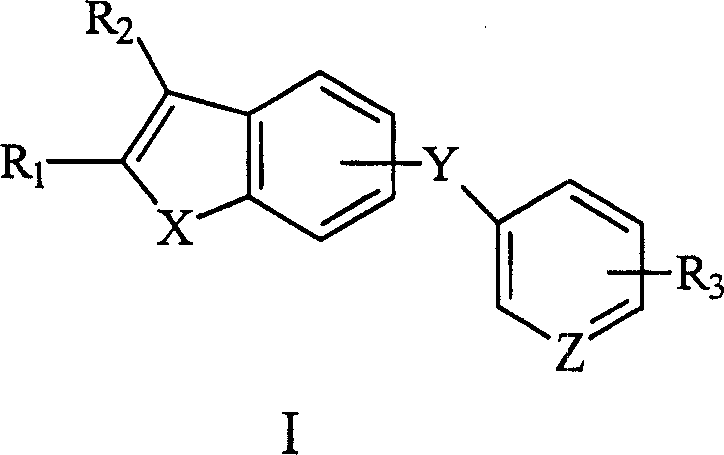
Antitumor compound and its prepn process
A compound and lower alkane technology, applied in the field of antitumor compounds and their preparation, can solve the problems of poor bioavailability, toxic and side effects, and difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
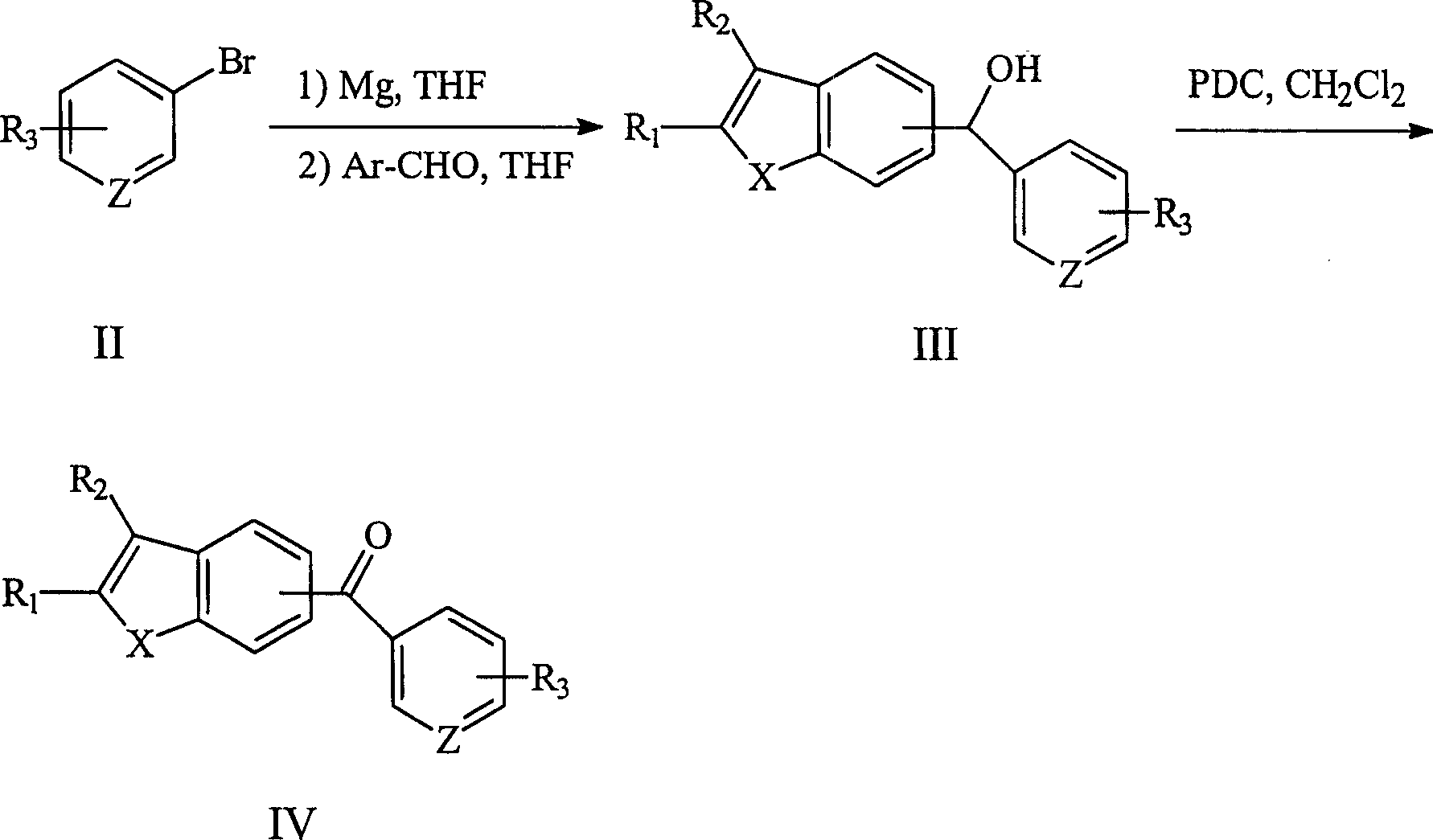
[0093] Example 1: (N-9-ethylcarbazole-3-substituted)-(3,4,5-trimethoxyphenyl)-methanol (123)
[0094] Add 2.5ml of anhydrous THF, magnesium strips (0.24g, 10mmol) and a few materials of iodine into the reaction flask, warm with an oil bath, and add about 1 / 4 of the total amount of 1-bromo-3,4,5- Trimethoxybenzene (2.47 g in total, 10 mmol) was dissolved in 10 ml of anhydrous THF solution. When the solution became colorless, the remaining THF solution of 1-bromo-3,4,5-trimethoxybenzene was slowly added dropwise to keep the solution slightly boiling. After the addition was complete, stirring was continued at room temperature for 1 hour. Then, the prepared THF solution of 3,4,5-trimethoxybenzenemagnesium bromide was slowly added to another 2.5ml of 9-ethyl-3-carbazole aldehyde (1.86g, 8.35mmol) without Aqueous THF solution, continue to react for 1 hour. Slowly add saturated NH at 0°C 4 Cl solution, and after 10 min, the solution was separated. The aqueous phase was extracted...
Embodiment 2
[0098] Example 2: (N-9-ethylcarbazole-3-substituted)-(3,4,5-trimethoxyphenyl)-methanone (124)
[0099] Pyridinium dichromate (PDC, 1.69 g, 4.5 mmol) was added to 30 ml of anhydrous CH containing compound 123 (1.17 g, 3 mmol) and molecular sieves 0.45 g at 0 °C 2 Cl 2 solution. After the addition was complete, it was stirred overnight at room temperature. The reaction solution was diluted with 50ml of ether and filtered. The filtrate was concentrated and separated by VLC to obtain off-white solid (0.90 g, yield 77%), which was compound 124, mp 125-126°C.
[0100] 1 H NMR (DMSO-d 6 ); δ1.35(t, J=7.2Hz, 3H), 3.78(s, 3H), 3.81(s, 6H), 4.39(q, J=7.2Hz, 2H), 7.07(s, 2H), 7.25 (dd, J=7.2, 7.8Hz, 1H), 7.42(dd, J=7.2, 6.9Hz, 1H), 7.68(d, J=8.1Hz, 1H), 7.73(d, J=8.4Hz, 1H) , 7.94 (dd, J=8.7, 1.5Hz, 1H), 8.31 (d, J=7.8Hz, 1H), 8.66 (d, J=1.2Hz, 1H).
[0101] 13 C NMR (DMSO-d 6 );
[0102] Elemental Analysis: C 24 h 23 NO 4 . Calculated: C, 74.01; H, 5.96; N, 3.60. Found: ...
Embodiment 3
[0103] Example 3: (2,6-dimethoxypyridine-3-substituted)-(N-9-ethylcarbazole-3-substituted)-methanol (135)
[0104] The compound 135 was prepared from the corresponding 3-bromo-2,6-dimethoxypyridine and 9-ethyl-3-carbazole aldehyde in the same manner as in Example 1. The product is light yellow solid, yield: 80%.
[0105] 1 H NMR (DMSO-d 6 ); δ1.27(t, J=6.9Hz, 3H), 3.81(s, 3H), 3.86(s, 3H), 4.38(q, J=6.9Hz, 2H), 5.70(d, J=1.2Hz , 1H), 6.00(d, J=1.2Hz, 1H), 6.38(d, J=8.1Hz, 1H), 7.1 5(dd, J=7.5, 7.5Hz, 1H), 7.37(dd, J=8.4 , 1.5Hz, 1H), 7.41(dd, J=7.8, 7.2Hz, 1H), 7.48(d, J=8.4Hz, 1H), 7.55(d, J=8.4Hz, 1H), 7.78(d, J =8.1Hz, 1H), 8.08(d, J=1.5Hz, 1H), 8.11(d, J=7.8Hz, 1H).
[0106] 13 C NMR (DMSO-d 6 );
[0107] Elemental Analysis: C 22 h 22 N 2 o 3 S 0.25H 2 O. Calculated: C, 72.01; H, 6.19; N, 7.64. Found: C, 71.96; H, 6.20; N, 7.47.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com