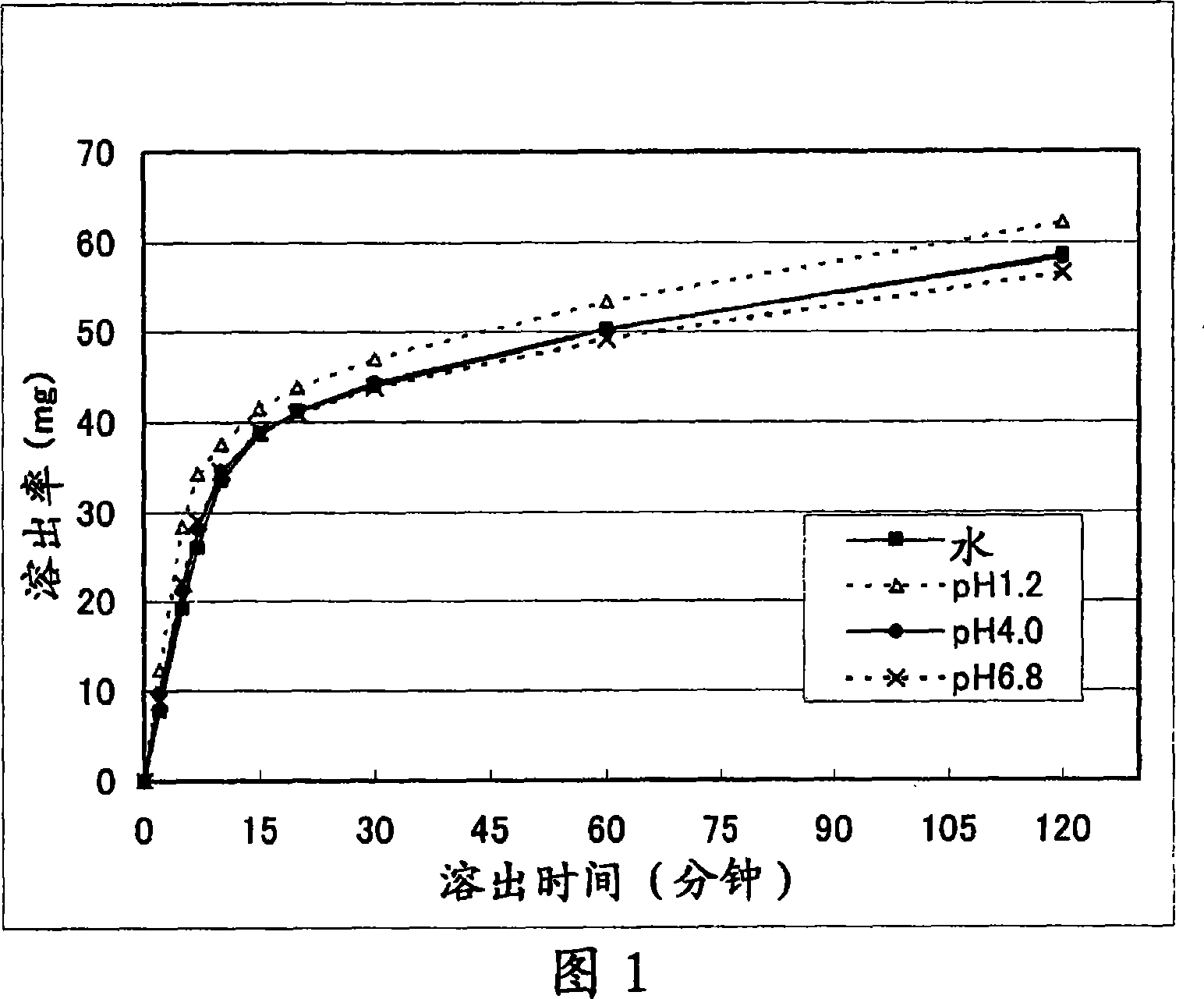
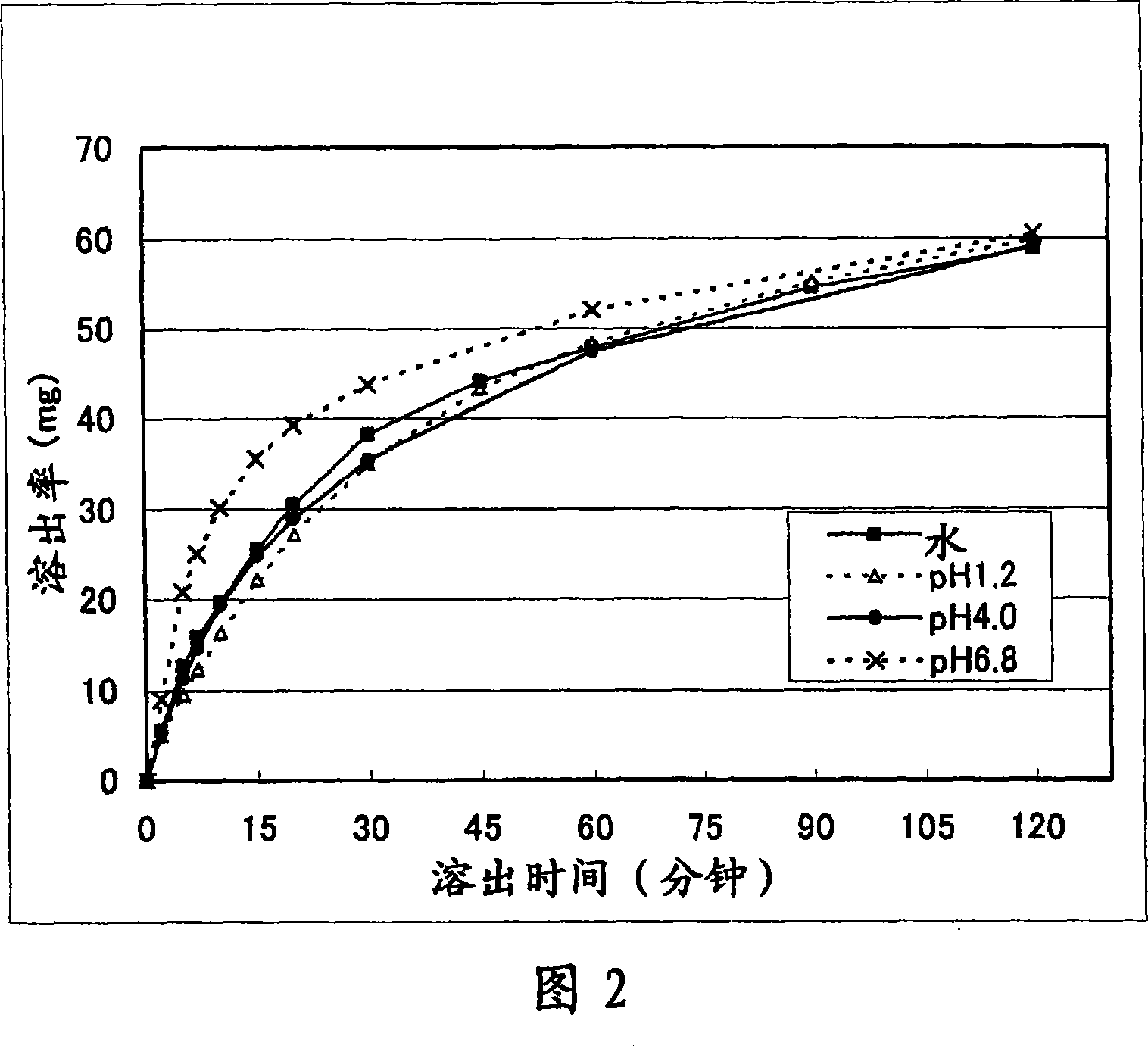
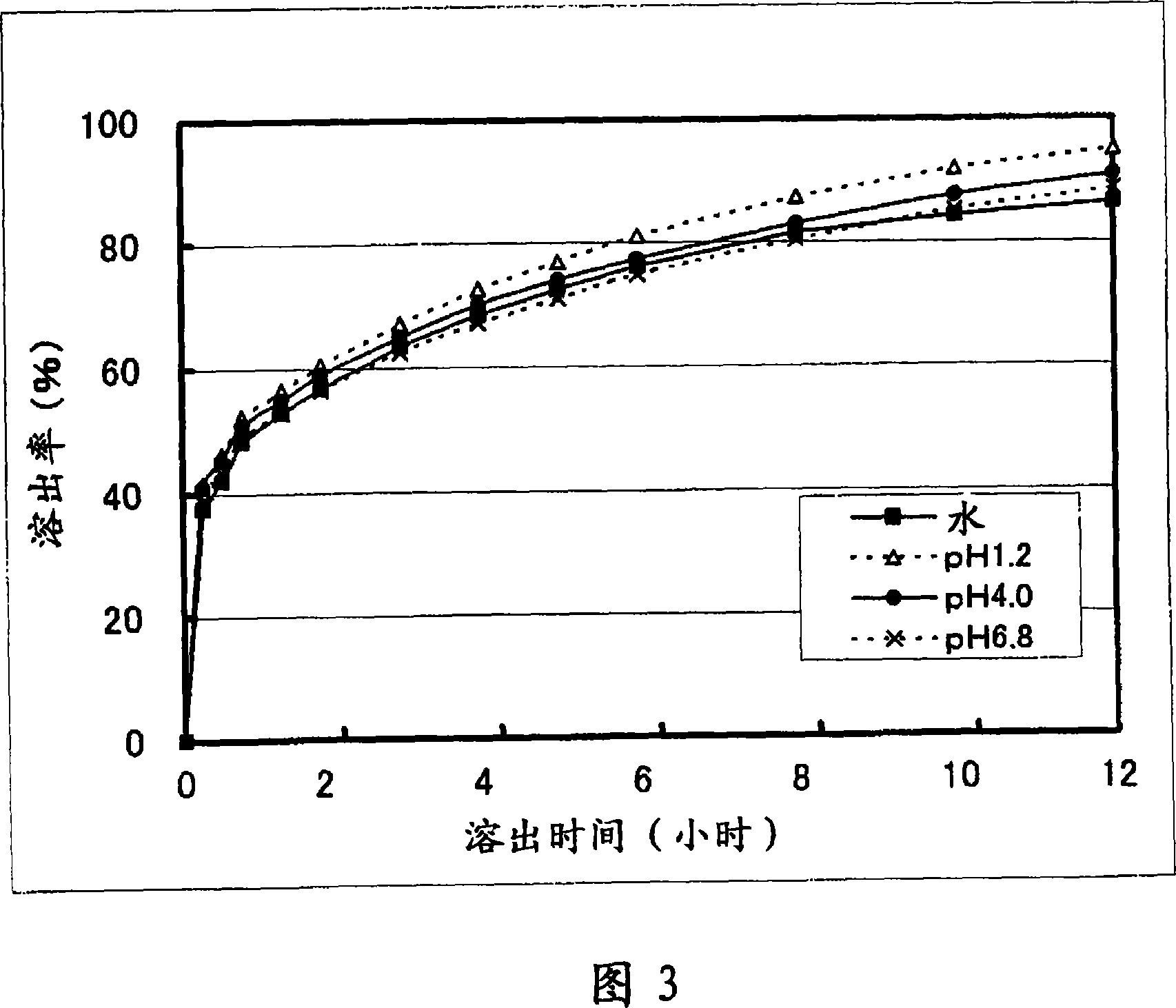
Solid pharmaceutical preparation
A technology of solid pharmaceutical preparations and additives, applied in the direction of drug combination, sugar-coated pills, pill delivery, etc., can solve the problem of small pH dependence and achieve high practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] The following examples are given to describe the present invention concretely, but the present invention is not limited to these examples.
[0026] Embodiment and comparative example: preparation method of tablet
[0027] Regarding the preparations of Examples prepared according to the formulation of the solid pharmaceutical preparation of the present invention and the preparations of Comparative Examples prepared according to the formulation of the solid pharmaceutical preparation of the present invention having different additives (refer to Japanese Patent Application No. 2004-288138), in Table 1 shows the compounding quantity of each contained component per 1 tablet. Each tramadol hydrochloride of the embodiment (tramadol hydrochloride content is 100, 75 and 50mg / tablet) and comparative example (tramadol hydrochloride content 100mg / tablet) of the composition shown in table 1 is produced by the following preparation method Multiple layers.
[0028]
Pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com