Use of BODIPY analog fluorescent reagent in biological large molecule marking
A biomacromolecule and fluorescent reagent technology, applied in the field of biomacromolecule labeling, achieves the effects of simple fluorescent labeling reaction, stable label product properties, and improved detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
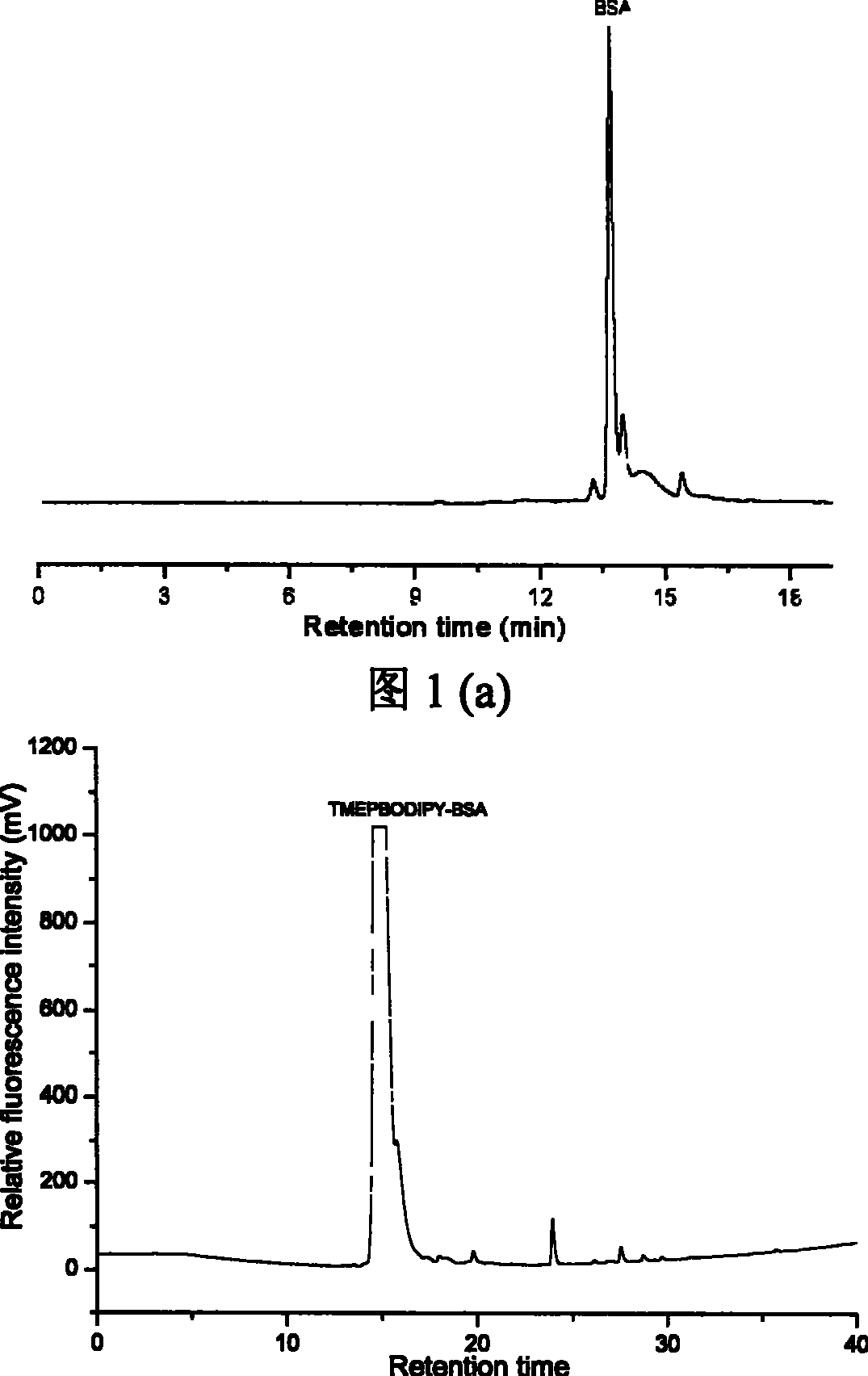
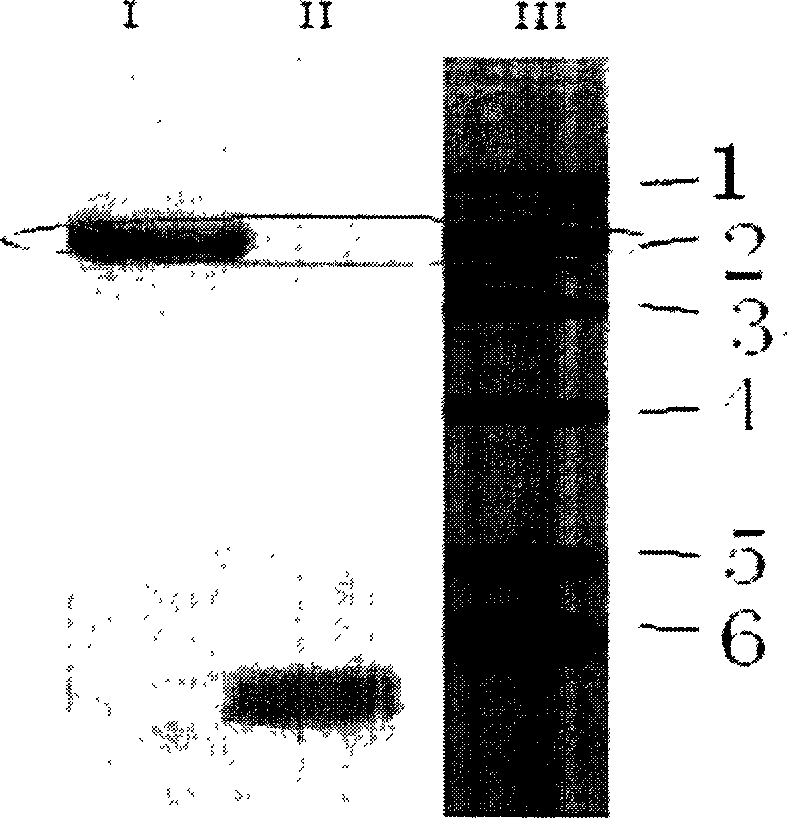
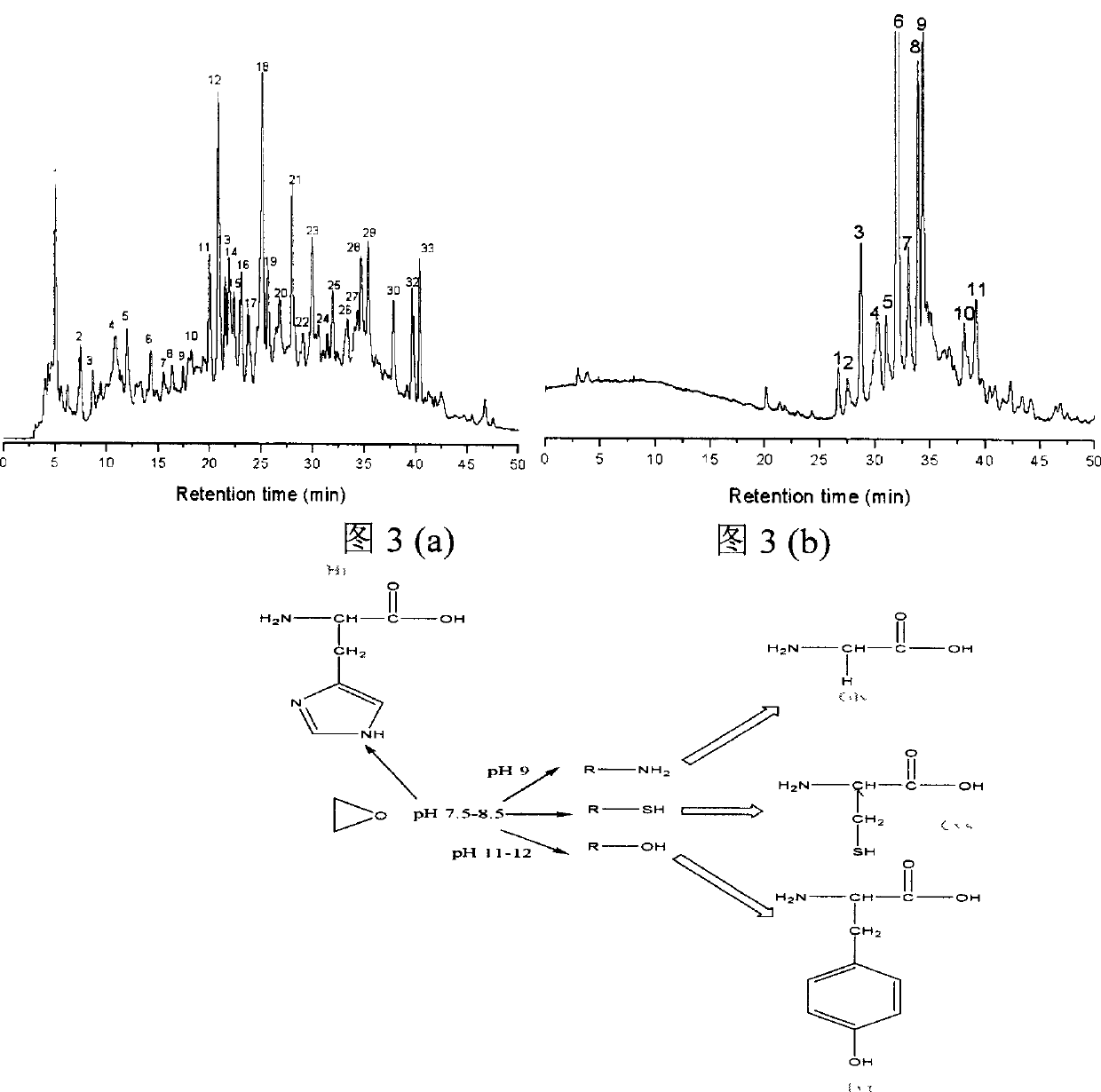
[0034] (1) The specific steps of the synthesis and labeling method of the fluorescent reagent TMEPBODIPY are as follows:
[0035] (1) First, 2,4-dimethylpyrrole and p-hydroxybenzaldehyde are used as raw materials to generate hydroxyl BODIPY (1,3,5,7-tetramethyl-8-phenyl-(4-hydroxyl)-difluoro Boronide-dipyrromethane). Add 200 mg of p-hydroxybenzaldehyde, 115 mg of 2,4-dimethylpyrrole and 1 mg of trifluoroacetic acid (Lewis acid) into a 500 mL round-bottomed flask containing 100 mL of dichloromethane. Electromagnetic stirring at 40°C for 10-12h. After the reaction is complete, evaporate the solvent to dryness, dissolve the residue in toluene, add 110 mg of chlorobenzoquinone, stir and react at room temperature for 2 hours, the solution turns dark brown, then add 242 mg of triethylamine and stir for 5 minutes, and immediately add 0.5 mL of boron trifluoride (including 263 mg of boron trifluoride) ether solution (47%, w / w), stirred and reacted at room temperature for 25-35 minut...
Embodiment 2
[0055] In the general formula [1], R represents the preparation method of the compound represented by the aryl group with an epoxy group,
[0056] The preparation of such compounds can be carried out by using aryl formaldehyde compounds (such as: p-hydroxybenzaldehyde) and 2,4-dimethylpyrrole under heating in a nitrogen water bath, using tetrachlorobenzoquinone as a dehydrogenating agent, and using triethyl Amine reaction terminator, finally add the ether solution of boron trifluoride to complete the complexation reaction.
[0057] The reaction formula is as follows:
[0058]
[0059] In the formula, R2 represents an aryl group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com