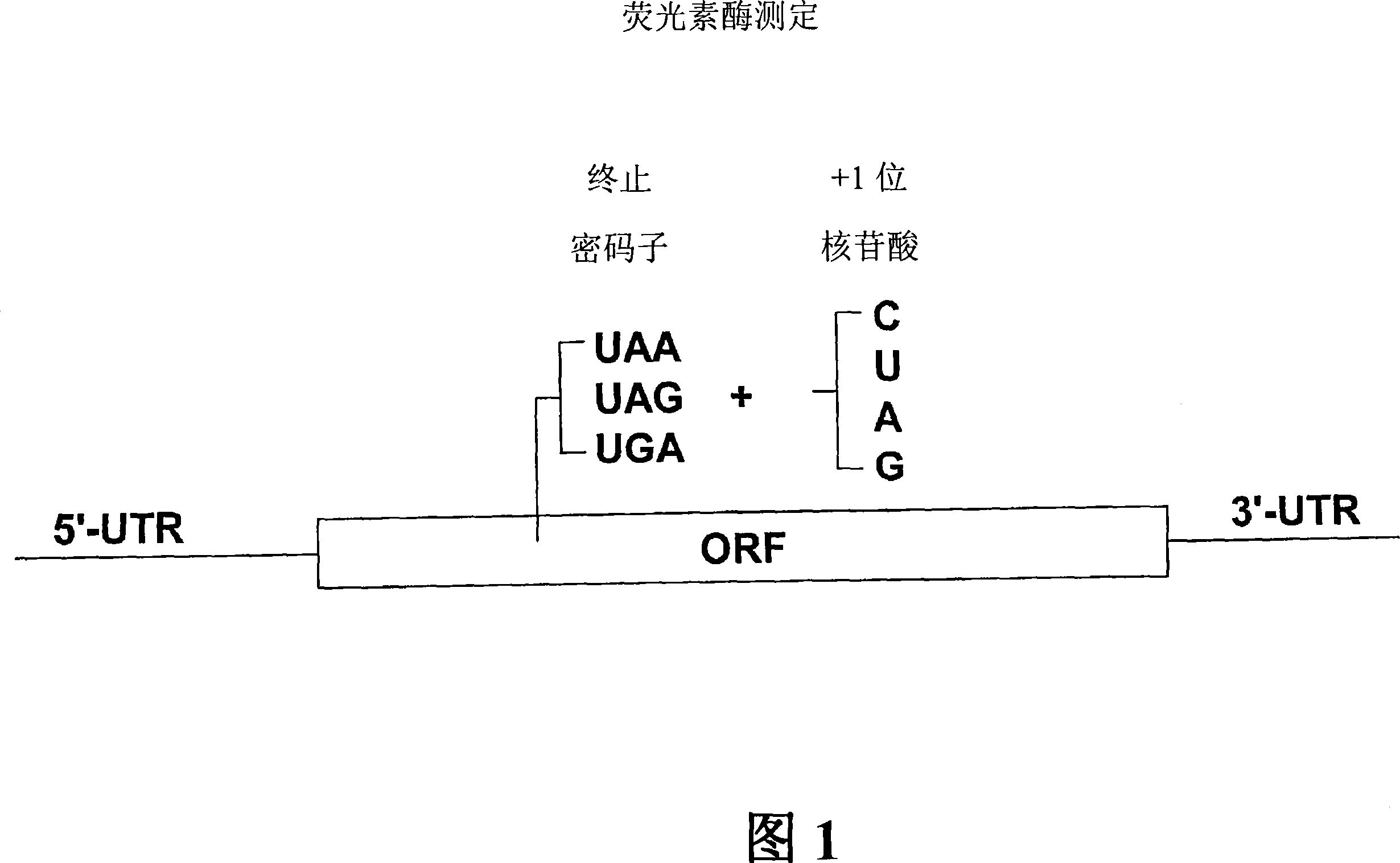
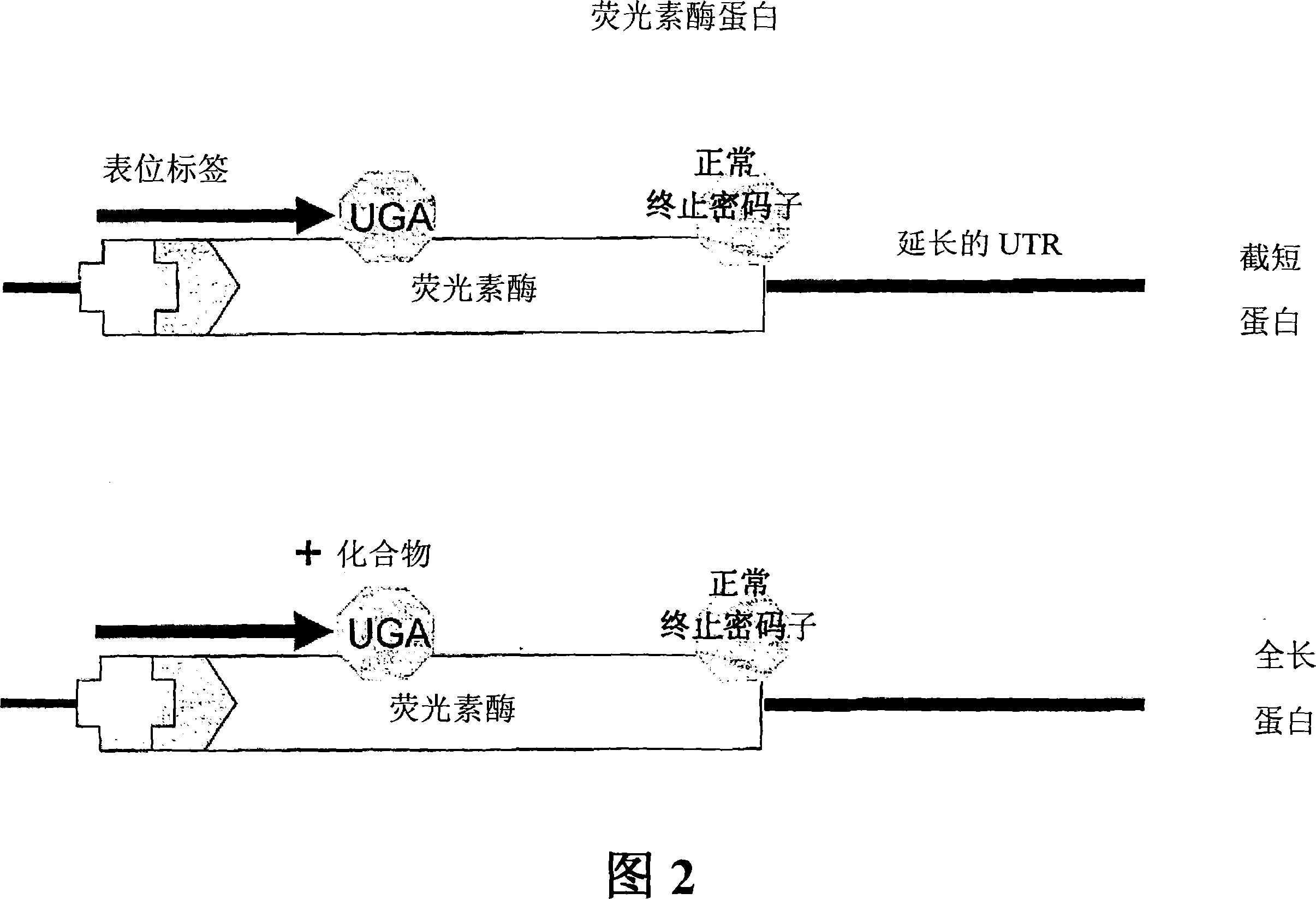
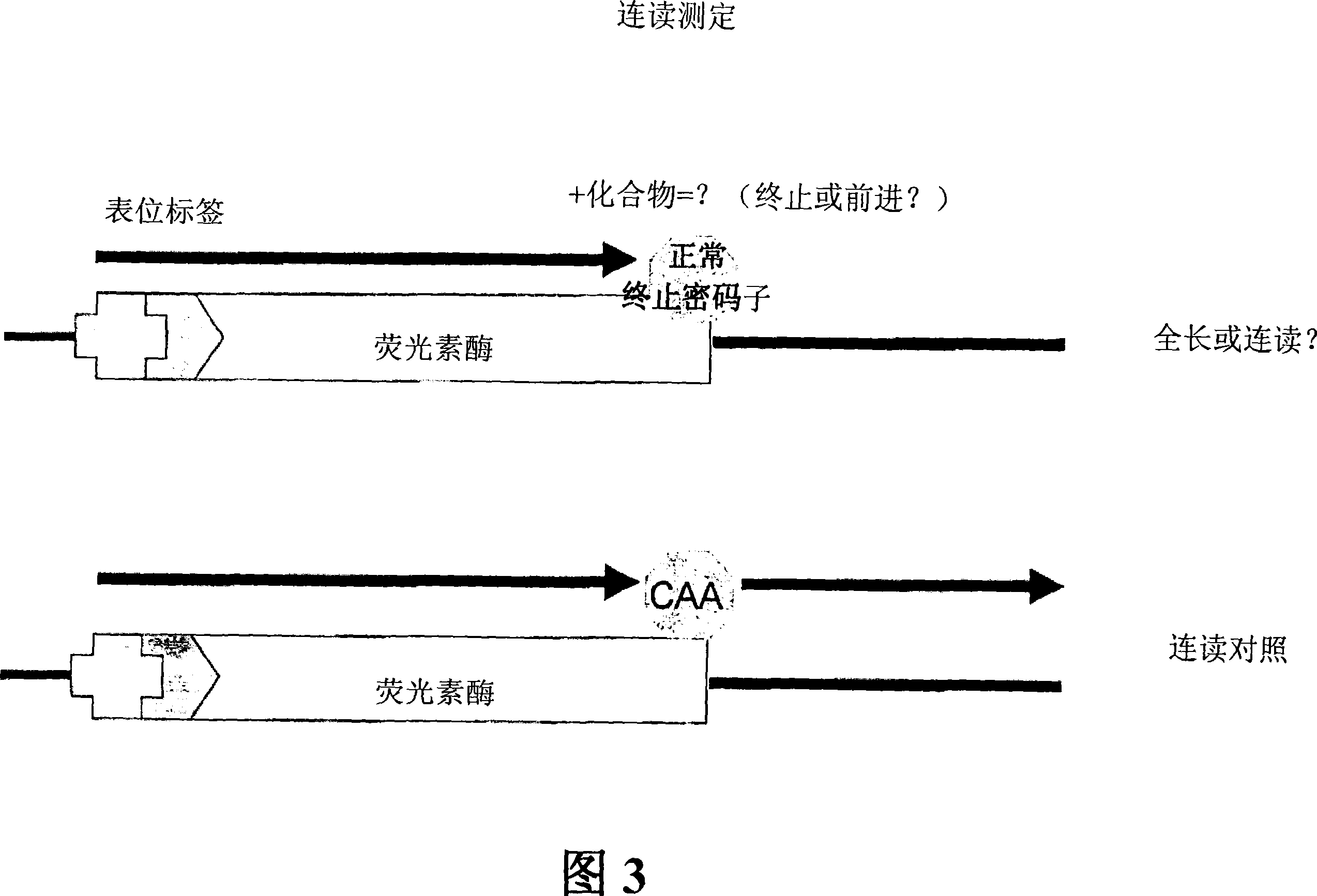
Compounds and their use for treating somatic mutation-related diseases
A compound, hydrate technology, applied in the treatment or prevention of diseases related to mRNA nonsense mutation, inhibiting premature translation termination related to mRNA nonsense mutation, can solve the problem of no public application of clitocine, no report of development of clitocine analogs or Derivatives etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0356] B. Preparation of Compounds of the Invention
[0357] The compounds of the invention may be prepared in any manner known in the art. For example, compounds of the present invention can be prepared according to the general methods described below. For example, certain 1,3,4-oxadiazoles of Formula 1 can be prepared as shown in Scheme A1 below:
[0358]
[0359] Route A1
[0360] According to Scheme A1, benzonitriles of structure Al can be converted to tetrazoles of structure A2 by treatment with eg sodium azide. Treatment of tetrazole A2 with an activated carboxylic acid such as an acid chloride or an acid activated with a dehydrating agent such as dicyclohexylcarbodiimide in a suitable solvent affords the 1,3,4-oxadiazole compound of formula 1-A. Suitable solvents include, but are not limited to, eg toluene or dichloroethane. The reaction can generally be carried out at a temperature in the range of 60-150°C.
[0361]In another embodiment...
Embodiment 1
[0555] Embodiment 1: the preparation of the compound of the present invention
Embodiment A
[0556] Example A: Preparation of 3-[5-(4-isopropylphenyl)-[1,3,4]oxadiazol-2-yl]benzoic acid (Compound No. 6)
[0557]
[0558] Step A: A suspension of methyl 3-cyanobenzoate (5.05 g, 31.4 mmol), sodium azide (3.06 g, 47.0 mmol) and triethylamine hydrochloride (6.47 g, 47 mmol) in 60 mL of toluene was heated Reflux for 12 hours, then cool to room temperature. The heterogeneous mixture was diluted with water and the phases were separated. The organic layer was extracted with saturated sodium bicarbonate, and the aqueous phases were combined and washed with EtOAc. After discarding the organic layer, the combined aqueous phases were acidified to about pH 2 with 6N hydrochloric acid, and the resulting thick paste was extracted with EtOAc (2x). The combined organic layers were washed with saturated NaCl, then dried and concentrated to give 5.30 g (83%) of methyl 3-(1H-tetrazol-5-yl)benzoate as a white solid: mp 180-181 °C; MS m / z 205.1[MH + ].
[0559] Step B: Methyl 3-(1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com