Matrix type sustained-release preparation containing basic drug or salt thereof
A technology of slow-release preparations and basic drugs, which is applied in the following fields of preparations, can solve the problems of reduced bioavailability and low release speed, and achieve the effects of reducing side effects, inhibiting the reduction of bioavailability, and reducing the risk of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] Another aspect of the present invention also provides a method for preparing a matrix-type sustained-release preparation, the method comprising the following steps: mixing a basic drug or its salt and at least one enteric polymer, the basic drug or its salt The solubility in 0.1N hydrochloric acid solution and 50 mM phosphate buffer at pH 6.0 is higher than that in alkaline aqueous solution at pH 8.0; the mixture obtained in the above mixing step is compression molded. The present invention also provides another method for preparing a matrix-type sustained-release preparation, which includes the following steps: mixing (1) a basic drug or its salt and (2) at least one enteric base, the basic drug or The solubility of its salt in 0.1N hydrochloric acid solution and neutral aqueous solution with pH 6.0 is 1 mg / mL or more, and the solubility in alkaline aqueous solution with pH 8.0 is 0.2 mg / mL or less, and in pH 6.8 The solubility in a neutral aqueous solution is at least 2 t...
Embodiment 1
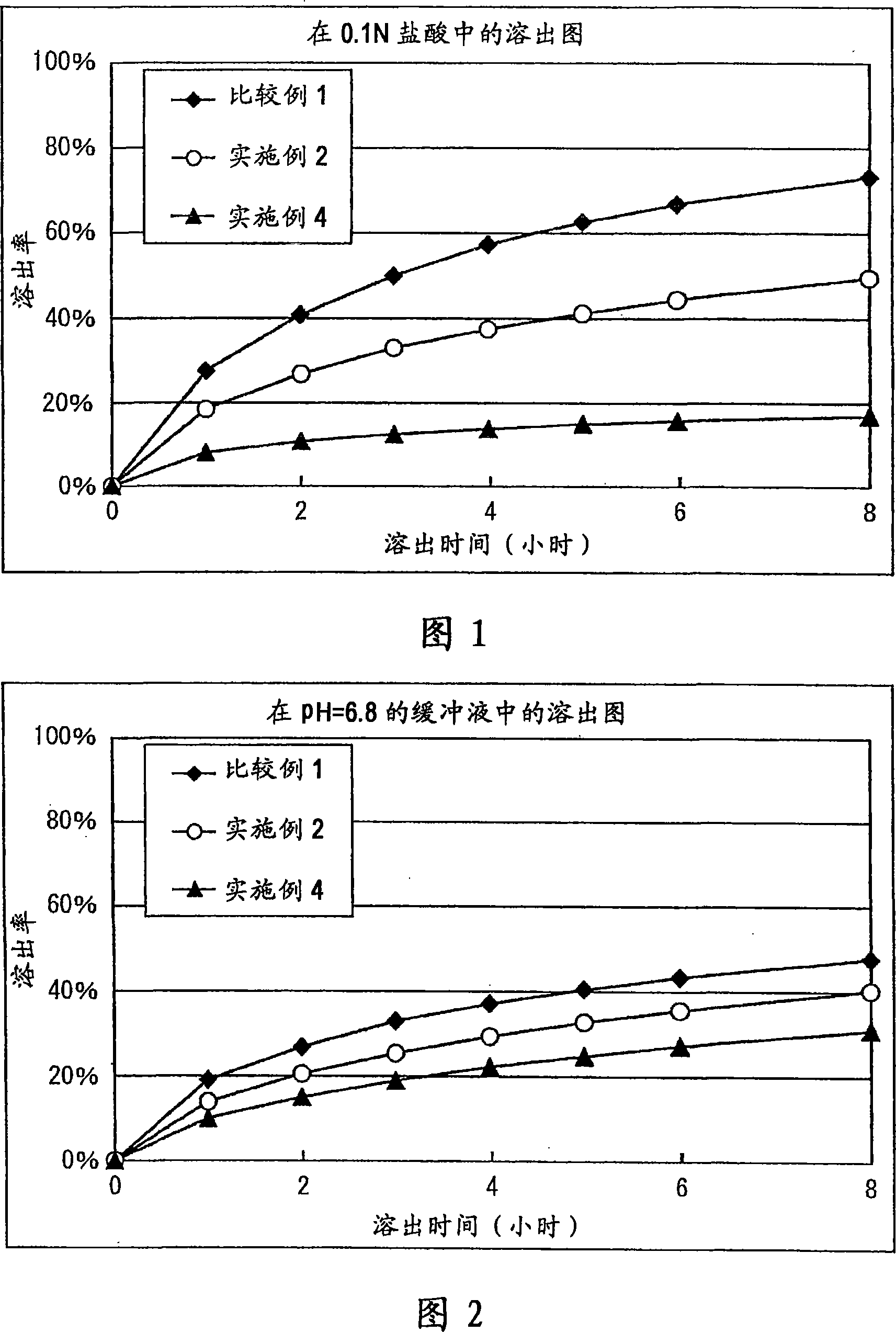
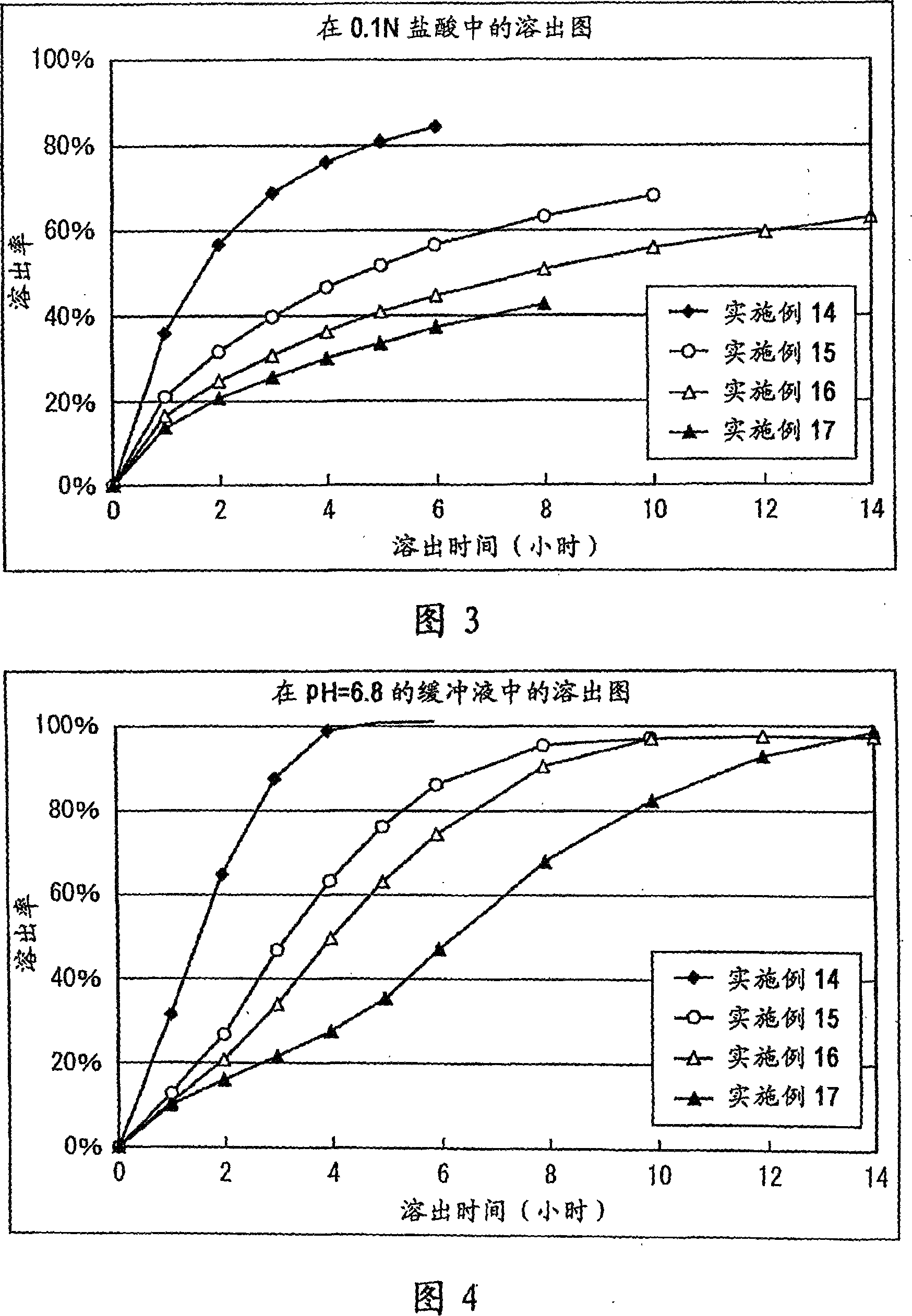
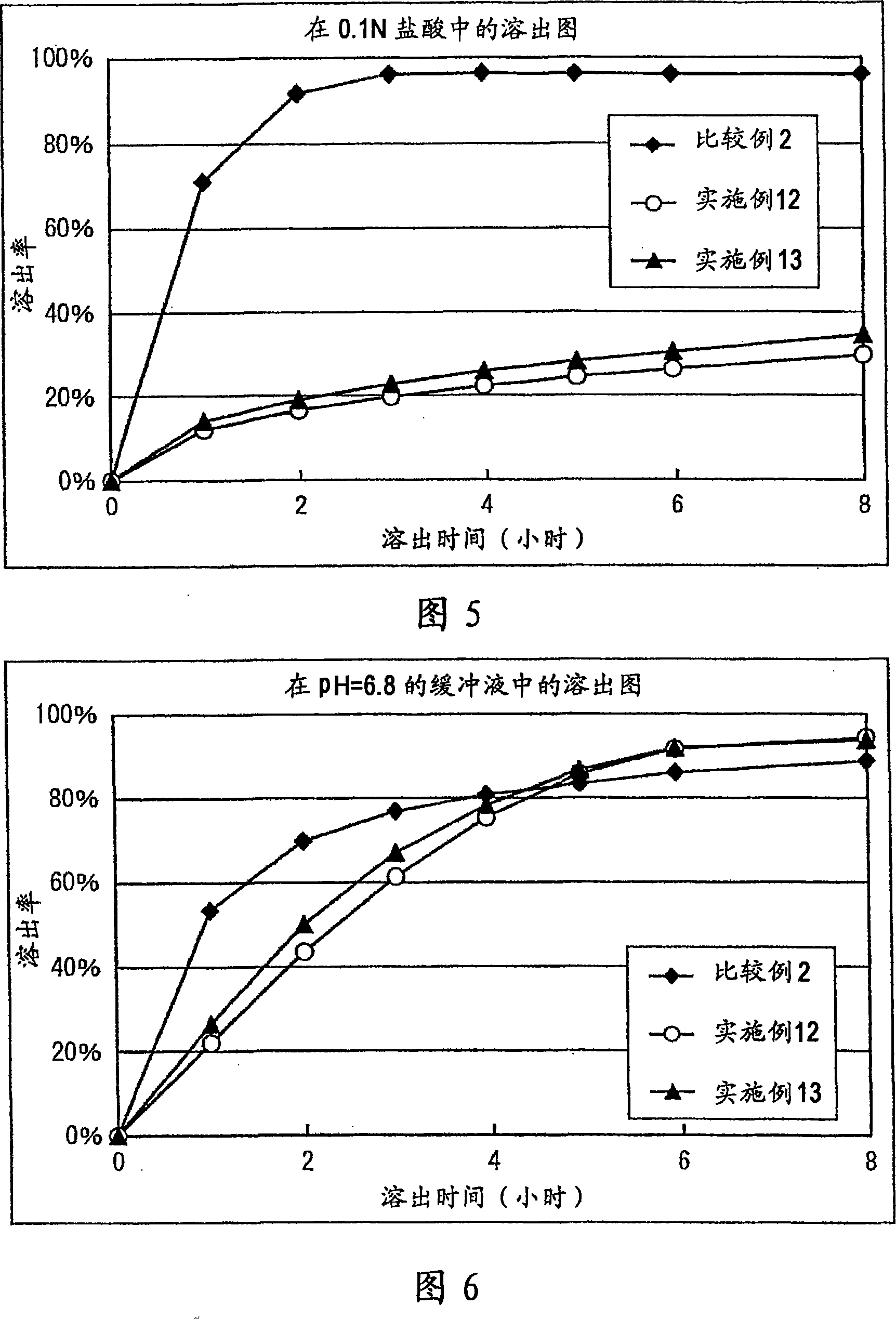
[0123] In a mortar, 300 mg of Donepezil Hydrochloride (Eisai), 1500 mg of Eudragit L100-55 (Rhm Gmbh & Co. KG), 1170 mg of lactose, and 30 mg of magnesium stearate (Mallinckrodt Baker, Inc.) were mixed. 200 mg of the above mixture was taken, and tablets were prepared using Autograph AG5000A (Shimadzu Corporation) to obtain tablets with a diameter of 8 mm containing 20 mg of donepezil hydrochloride. The results of the dissolution test are shown in Table 1.
Embodiment 2
[0125] Mix 300mg Donepezil Hydrochloride (Eisai), 750mg Ethocel 10FP (Ethylcellulose, Dow Chemical Company), 750mg Eudragit L100-55 (Rhm Gmbh&Co.KG), 1170mg lactose, 30mg hard fat in a mortar. Magnesium acid (Mallinckrodt Baker, Inc.). 200 mg of the above mixture was taken, and tablets were prepared using Autograph AG5000A (Shimadzu Corporation) to obtain tablets with a diameter of 8 mm containing 20 mg of donepezil hydrochloride. The results of the dissolution test are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com