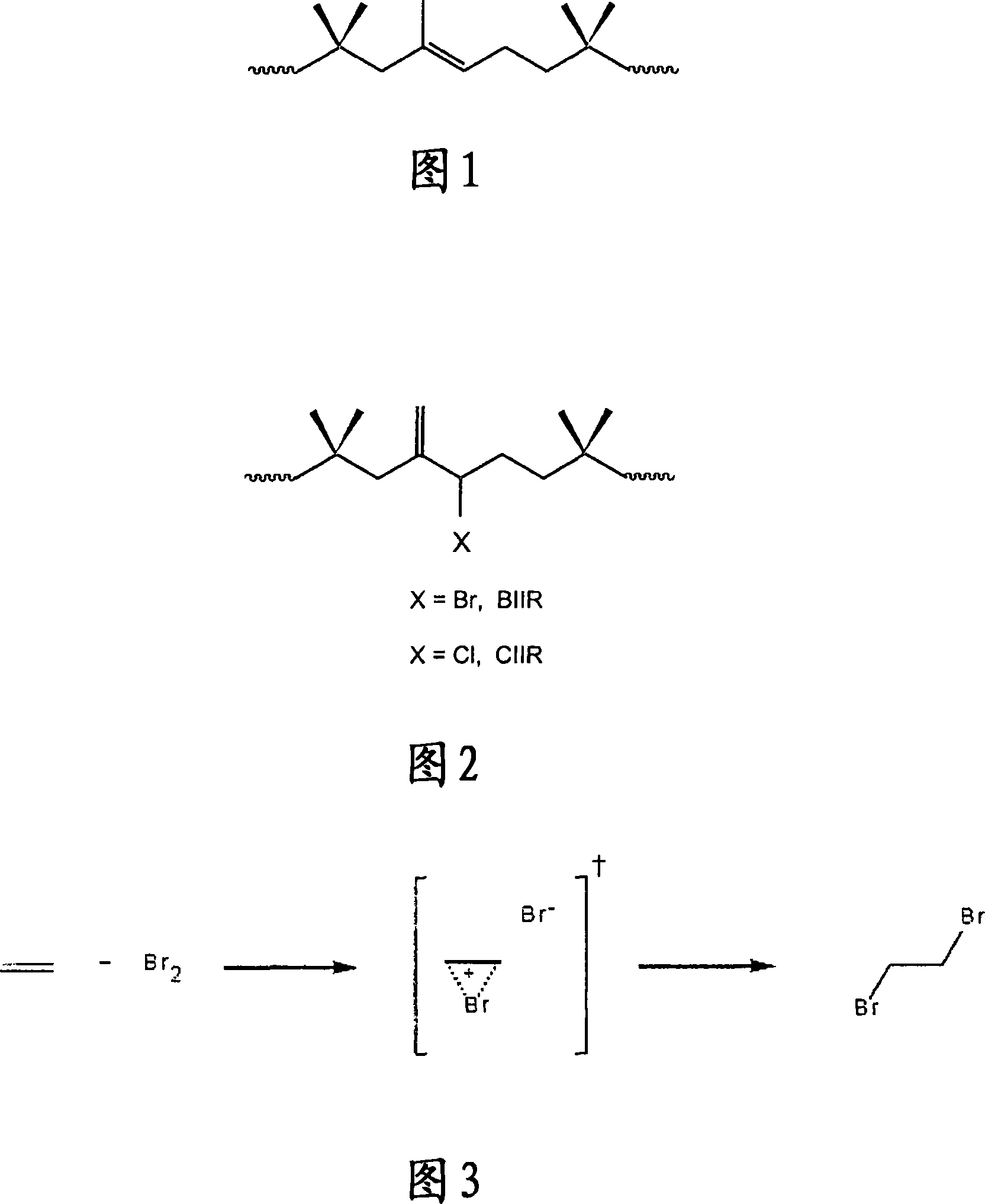
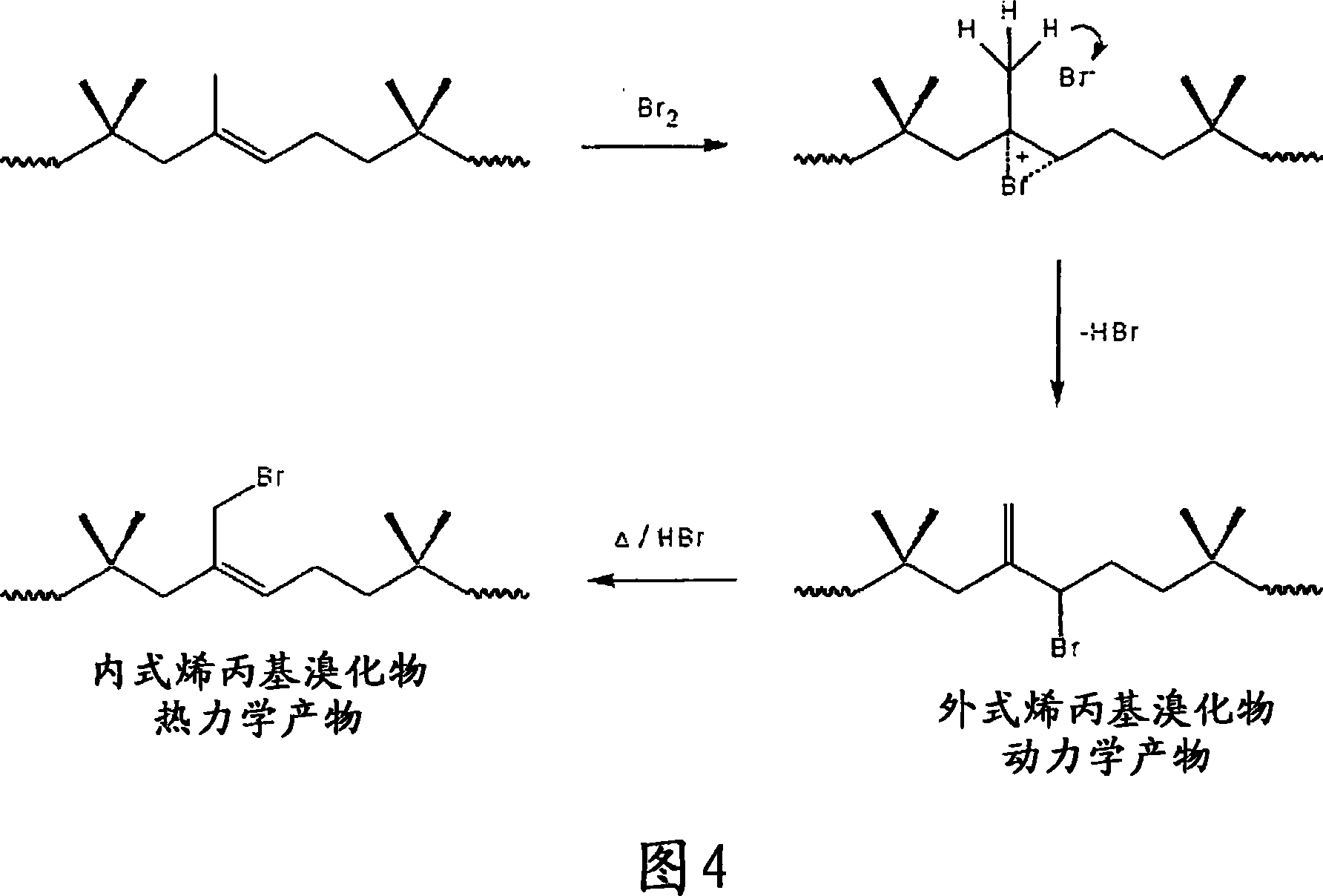
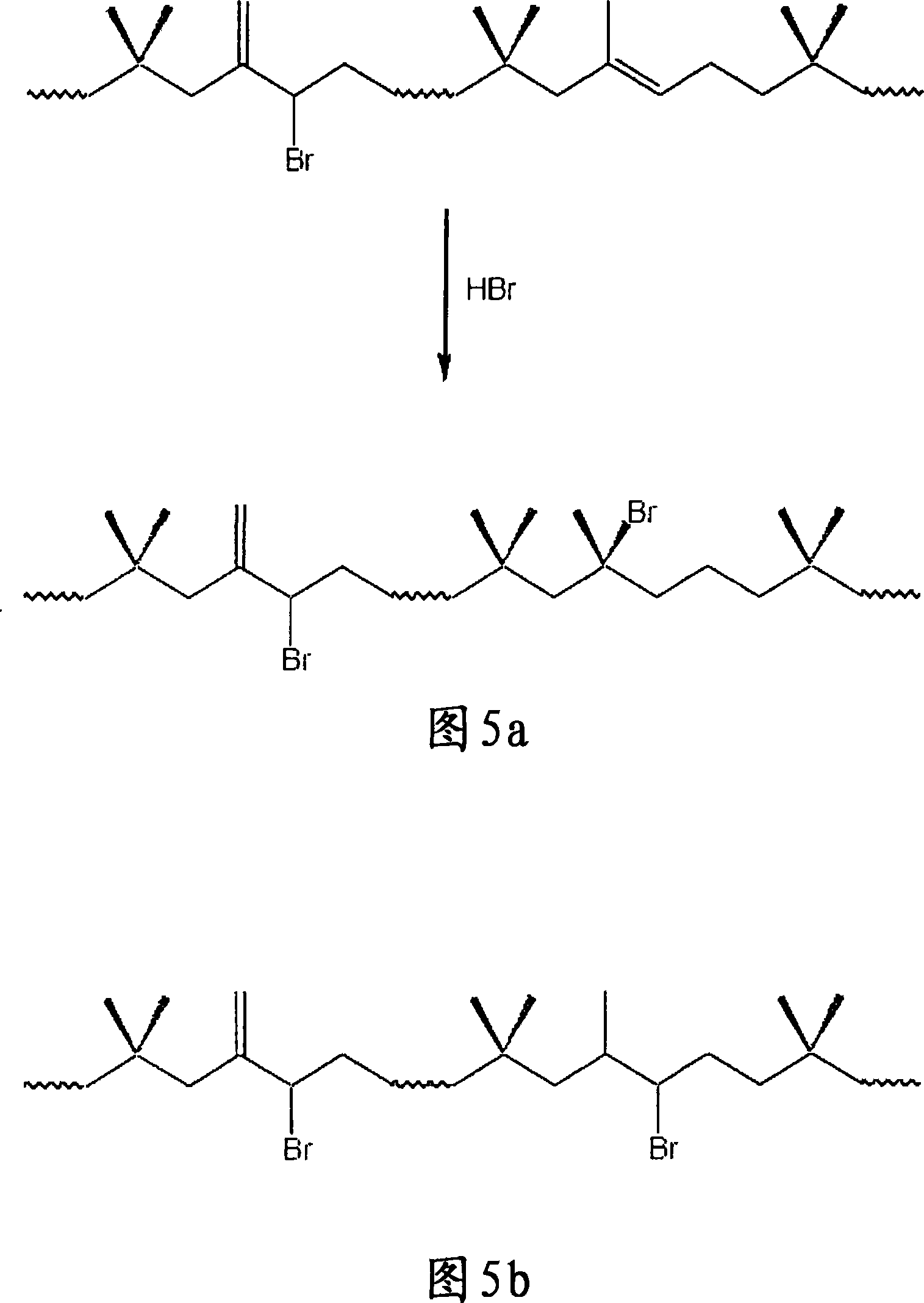
Method for halogenation of butyl rubber without using acid neutralizing agent
A technology for halogenating butyl rubber and butyl rubber, applied in the field of halogenation of butyl rubber, can solve the problems of no enlightenment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Bromination of RB301 (with water and caustic). To a solution of RB301 (50 g, 1.6 mol% 1,4-isoprene) in 600 mL hexane was added 45 mL of water. To this mixture was added 0.63 mL of elemental bromine with rapid stirring. After 5 minutes, the reaction mixture was neutralized by introducing a caustic solution prepared by mixing 6.5 mL of aqueous 1.0 M NaOH in 500 mL of water. Immediately following neutralization, 4 ml of stabilizer solution (3.75 g of epoxidized soybean oil and 0.045 g of Irganox 1076 in 100 ml of hexane) were fed to the reaction mixture. The rubber was isolated by steam condensation and dried to constant weight with the aid of a 6" x 12" two-roll mill operating at 100°C. use 1 H nuclear magnetic resonance spectrometer (CDCl 3 ) to measure the microstructure of the obtained material, and the results are listed in Table 1.
Embodiment 2
[0048] Example 2: Bromination of RB301 (without water and caustic). To a solution of RB301 (50 g, 1.6 mol% 1,4-isoprene) in 600 mL of hexane was added 0.63 mL of elemental bromine under rapid stirring. After 5 minutes, 4 ml of stabilizer solution (3.75 g of epoxidized soybean oil and 0.045 g of Irganox 1076 in 100 ml of hexane) were fed to the reaction mixture. The rubber was isolated by steam condensation and dried to constant weight with the aid of a 6" x 12" two-roll mill operating at 100°C. use 1 H nuclear magnetic resonance spectrometer (CDCl 3 ) to measure the microstructure of the obtained material, and the results are listed in Table 1.
Embodiment 3
[0049] Example 3: Bromination of RB402 (without water and caustic). To a solution of RB402 (50 g, 2.0 mol% 1,4-isoprene) in 600 mL of hexane was added 0.63 mL of elemental bromine under rapid stirring. After 5 minutes, 4 ml of stabilizer solution (3.75 g of epoxidized soybean oil and 0.045 g of Irganox 1076 in 100 ml of hexane) were fed to the reaction mixture. The rubber was isolated by steam condensation and dried to constant weight with the aid of a 6" x 12" two-roll mill operating at 100°C. use 1 H nuclear magnetic resonance spectrometer (CDCl 3 ) to measure the microstructure of the obtained material, and the results are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com