Solanesyl polyamine derivative, preparation and application thereof
A technology of amine derivatives and solanes, which is applied in the field of solanesyl polyamine derivatives, preparation and application, can solve the problem of low anti-tumor activity, achieve strong anti-tumor activity, high reaction yield, and easy operation Effect
Inactive Publication Date: 2010-10-13
HENAN UNIVERSITY
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In recent years, researches on the antitumor activity of solanesol derivatives have become increasingly active. At present, there have been many reports on solanesol derivatives with different structures. However, the antitumor activities of such derivatives have been published. Very low, cannot be used as a drug lead compound for in-depth research
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
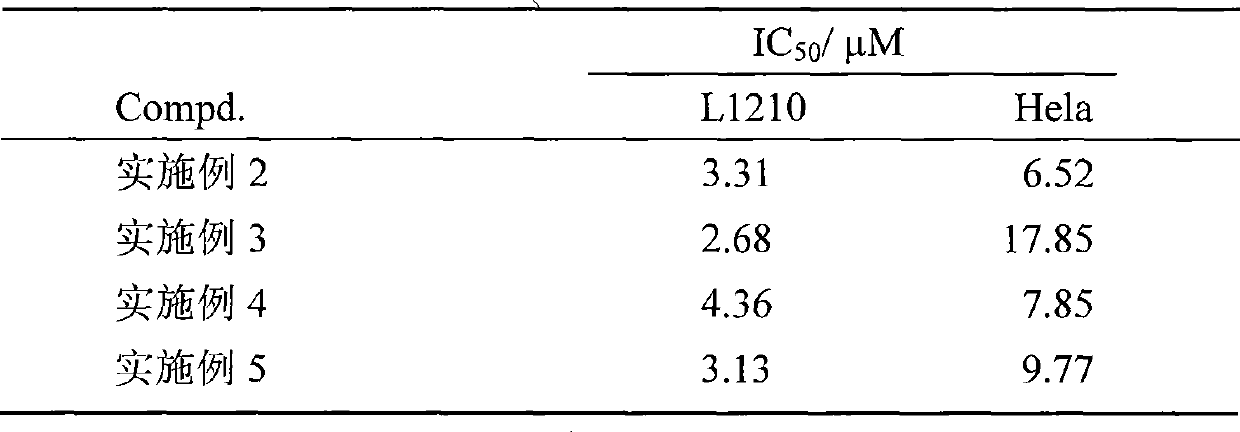
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
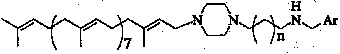
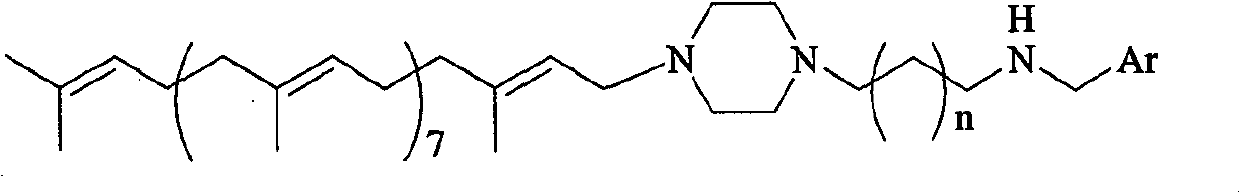
The invention discloses solanesyl polyamines derivatives which are compounds with the following common structural formula, wherein, n is equal to 0-2, Ar is phenyl, substitutive phenyl, 1-naphthyl, 2-naphthyl, 9-anthryl or tricyclic aryl containing N,S atoms. The preparation method is as follows: dissolve N-solanesyl-N amine alkyl piperazine in organic solvent; add aromatic aldehyde and make the mixture react for 2-24 hours under the temperature of 0-20 DEG C; steam the mixture to get rid of the solvent and then add reducer when bathing the mixture in ice water and let the mixture react for 2-12 hours under room temperature; process the products and separate the products by column chromatography to get the solanesyl polyamines derivative. The invention provides a series of new solanesyl polyamines derivatives which are the homologous derivatives of natural products, maintain the original all-trans stereo configuration of the solanesol unit and can be used as anti-neoplastic drugs and primer for the anti-neoplastic drugs. The preparation method of the invention is of simple operation with moderate condition and high reaction yield and with great market potential, thus is easy to beindustrialized.
Description
technical field The invention belongs to the technical field of pharmaceutical compounds, preparation and application thereof, and relates to a solanesyl polyamine derivative, preparation and application thereof. Background technique Solanesol naturally exists in tobacco leaves and potato leaves. It is a tetrasesquiterpene alcohol with nine isoprene units and is a natural polyterpene compound in all-trans stereo configuration. In the living body, it is an indispensable biosynthetic precursor of many isoprenoid complexes, isoprenyl proteins and carbohydrate compounds. And it has an important biochemical role as a component of biofilm and cells. Many derivatives obtained by structural modification based on solanesol have exact and important physiological functions. In recent years, researches on the antitumor activity of solanesol derivatives have become increasingly active. At present, there have been many reports on solanesol derivatives with different structures. However,...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D295/125A61K31/495A61P35/00
Inventor 王超杰王建红赵瑾甘莹
Owner HENAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com