Preparation method for octacosanol
A technology of stearyl alcohol and stearyloxy, applied in the field of organic synthesis, can solve the problems of poor atom economy and low efficiency, and achieve the effects of low toxicity, simple process and abundant sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
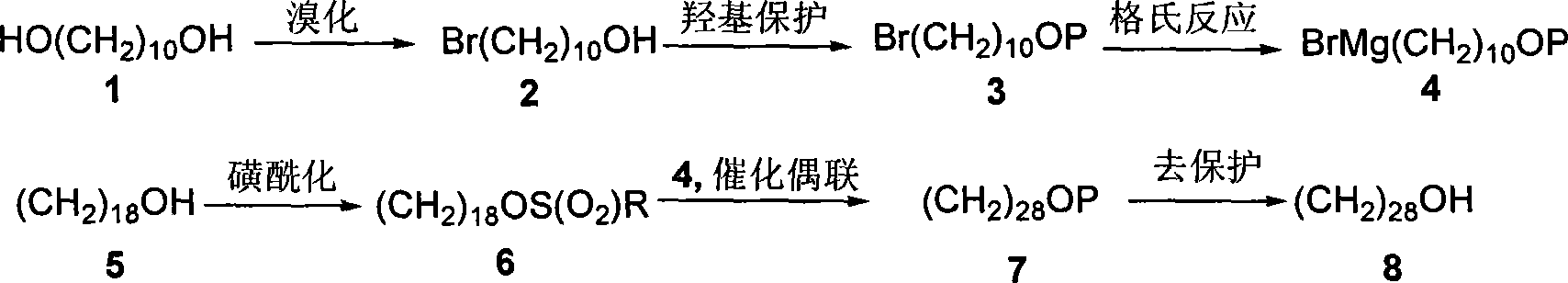
[0032] Step 1: Preparation of 10-bromo-n-decyl alcohol (2)
[0033] Add 1,10-decanediol (1) (11.6g, 67mmol) and toluene (200mL) into a 500mL three-necked flask equipped with a water separator, flow condenser and constant pressure dropping funnel, and use The oil bath was heated to reflux. After the solid was dissolved, 40% HBr (16.3 mL, 80 mmol) was slowly added dropwise from the dropping funnel, and the water generated by the reaction was separated from the water separator. After the conversion of (1) is complete, cool to room temperature and transfer to a separatory funnel, wash with saturated sodium bicarbonate (100mL), (100mL) and saturated sodium chloride (100mL) successively, dry over anhydrous sodium sulfate, and filter The toluene was distilled off under reduced pressure, and the crude product was subjected to flash column chromatography or distillation under reduced pressure to obtain 10-bromo-n-decanol (2) (14.3 g, 90%) as an oily liquid. IR (film, cm -1 )v max : ...
Embodiment 2
[0043] Similar to Example 1, the difference is that the solvent used in step 2 is dichloromethane, and the amount of p-toluenesulfonic acid is 0.2 molar multiples of (2).
Embodiment 3
[0045] Similar to Example 1, the difference is that the solvent used in step 4 is anhydrous ether, and the concentration of 2-(10-bromo-n-decyloxy)-tetrahydropyran (3) in anhydrous ether is 0.2mol / L, the temperature of the coupling reaction is -40°C, the amount of cuprous iodide is 0.45 mole multiple of (3), and the reaction time after adding octadecylsulfonate (6, R=Ts) is 2h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com