Combined chemical modified endomorphin-1 and method for preparing same
An endomorphin and compound technology, which is applied in the field of combinatorial chemically modified endomorphin-1 and its preparation, can solve the problems of rare analgesic analogs, short duration of analgesia, lack of oral activity and the like, Achieve the effects of improving bioavailability, prolonging analgesic action time, and simplifying synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
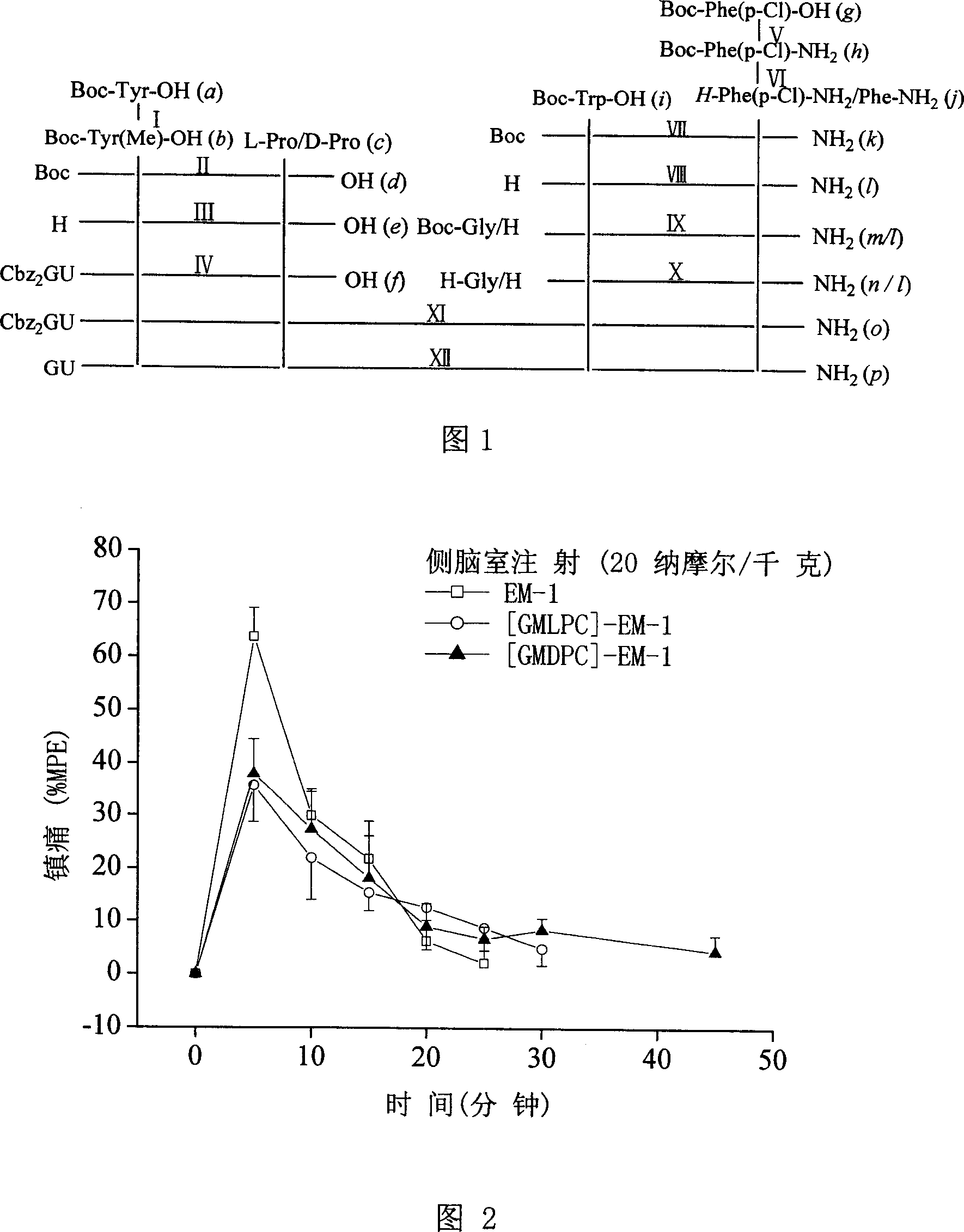
[0036] Example 1: Synthesis of Analogue [GMLPC]-EM-1
[0037] Reaction I: Synthesis of Boc-Tyr(Me)-OH(b)
[0038] Compound a (Boc-Tyr-OH) and 4 times the amount of NaH were respectively dissolved in anhydrous THF, protected by Ar, and the solution of compound a was slowly added dropwise to NaH / THF under stirring in an ice-salt bath. After reacting at -15°C for 30 min, excess CH3I was slowly added dropwise to the reaction system, and stirred overnight at room temperature. After the reaction is complete, extract the reaction with ice water at 0°C, concentrate THF under reduced pressure, leave the aqueous phase at the bottom of the bottle, add a mixture of ether / water (1:3), the product remains in the aqueous phase, and saturate with NaHCO 3 Wash the ether layer twice, combine with the previous aqueous phase, adjust the pH value of the aqueous phase to 3-4 with 20% citric acid, extract the aqueous phase with ethyl acetate three times, combine the ethyl acetate layers, and wash w...
Embodiment 2
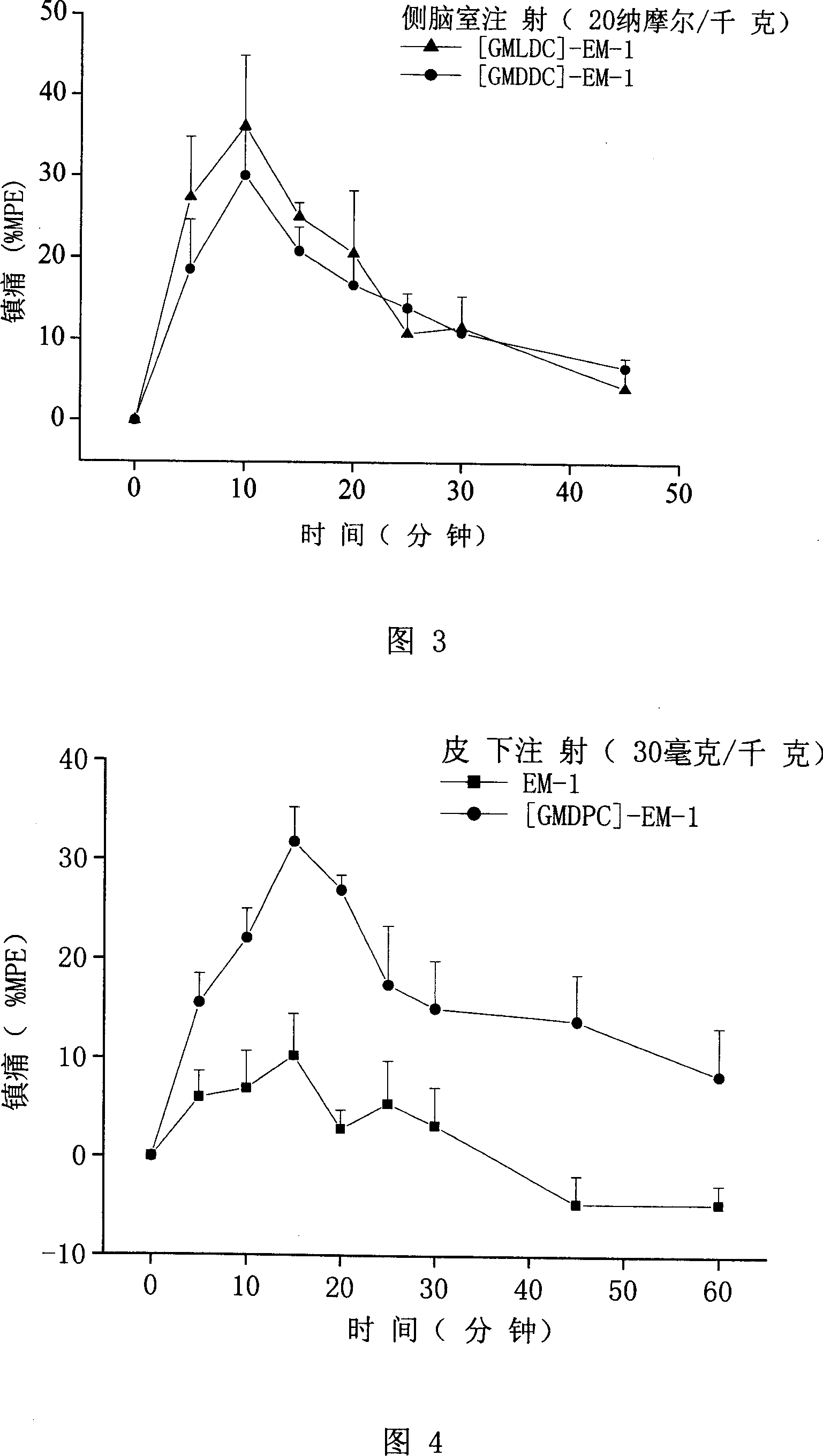
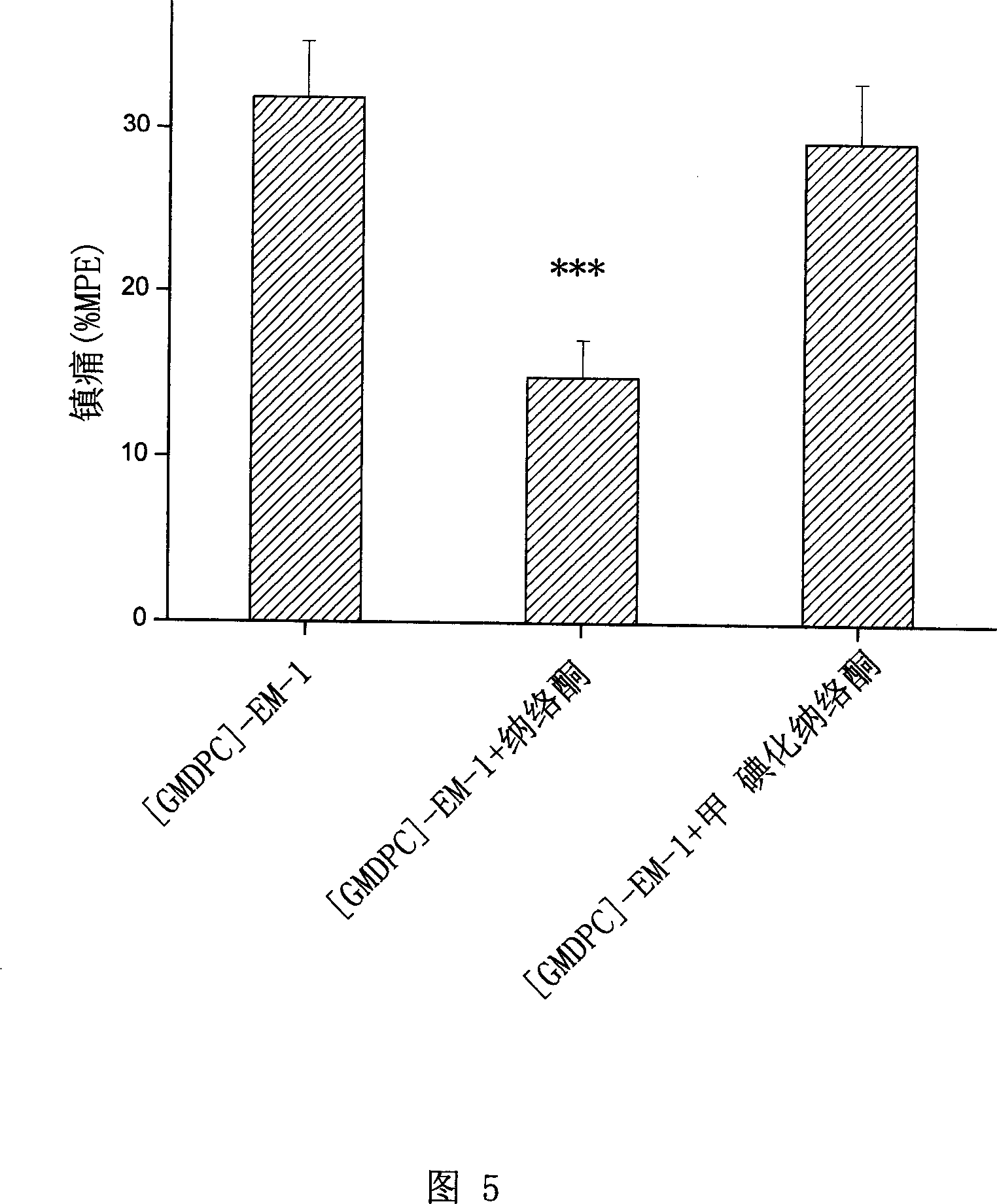
[0059] Example 2: Synthesis of Analogue [GMDPC]-EM-1
[0060] Reaction I: See Example 1.
[0061] Reaction II: Synthesis of Boc-Tyr(Me)-D-Pro-OH(d)
[0062] Compound b and a 1.1-fold excess of HOSu were dissolved in anhydrous THF, and after stirring in an ice bath for 10 min, a pre-cooled 1.1-fold excess of DCC / THF solution was slowly added dropwise, reacted in an ice bath for 30 min, then removed the ice bath, and reacted at room temperature for 6 h. The DCU precipitate was removed by vacuum filtration to obtain the activated ester [Boc-Tyr(Me)-OSu] solution of compound b. Under ice-bath stirring, dropwise add the NaHCO of the compound c (D-Pro) of equimolar amount to the filtrate 3 solution (pH=8-9), reacted overnight. After the reaction was complete, concentrate under reduced pressure to remove THF, add a large amount of ethyl acetate to dissolve the residue, wash with 5% citric acid and saturated NaCl three times, dry over anhydrous sodium sulfate, and concentrate. Pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com