Combined chemical modified endomorphin-1 and method for preparing same
An endomorphin and peptide fragment technology, which is applied in the field of combinatorial chemically modified endomorphin-1 and its preparation, can solve the problems of rare analgesic analogs, short analgesic duration, poor enzymatic stability, etc. It can improve the physical and chemical properties, prolong the analgesic effect, and improve the stability of enzymatic hydrolysis.
Active Publication Date: 2010-09-08
LANZHOU UNIVERSITY
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, EM-1 also has disadvantages similar to other endogenous opioid peptides, such as short duration of analgesia, lack of oral activity, poor enzymatic stability, rapid degradation in blood and brain, and short half-life. The presence of the BBB also hinders their access to the CNS to exert their effects, thus limiting the use of endomorphins as therapeutic drugs
Although many endomorphin analogues have been synthesized so far, there are few analogues that can be injected into the CNS to produce analgesia
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
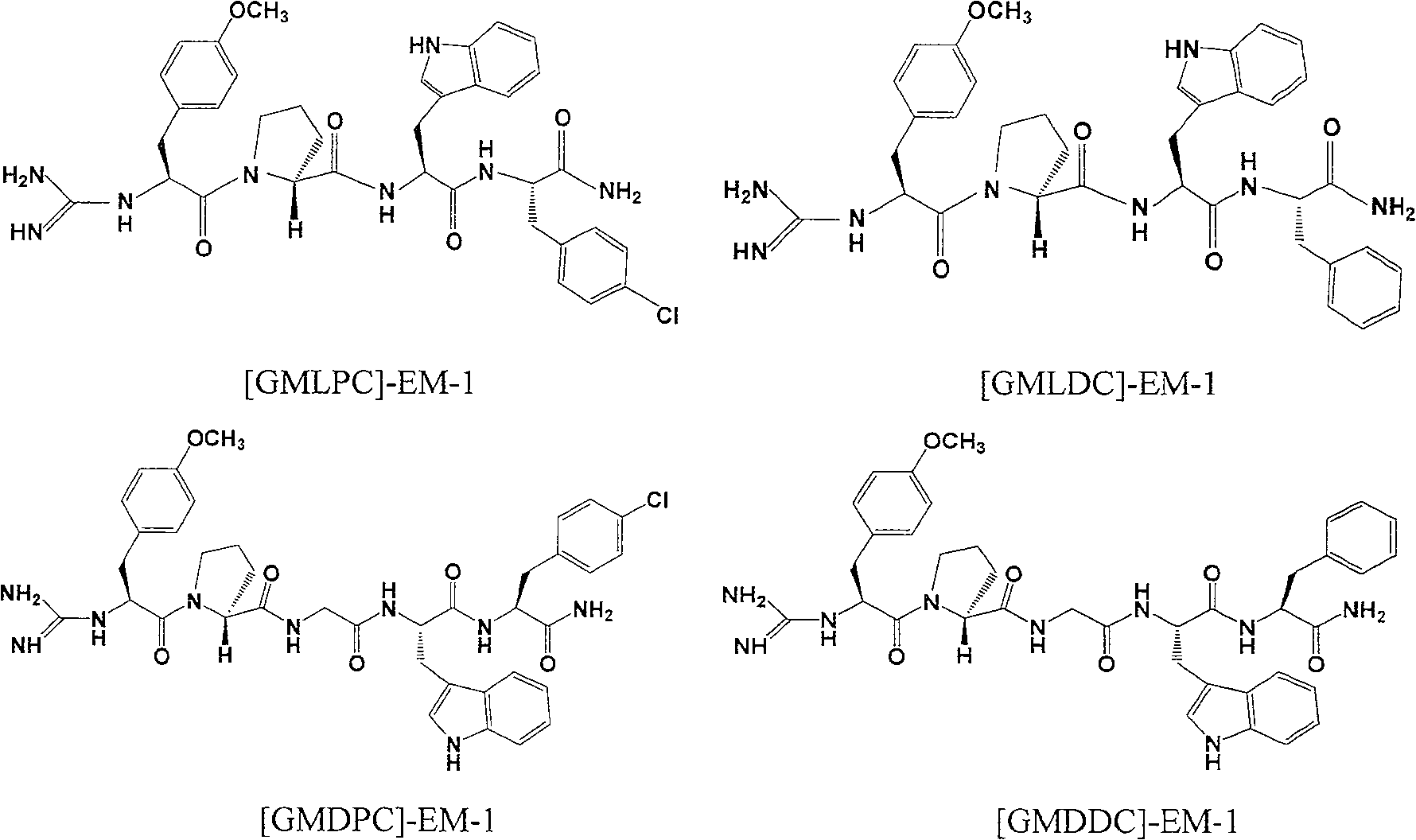
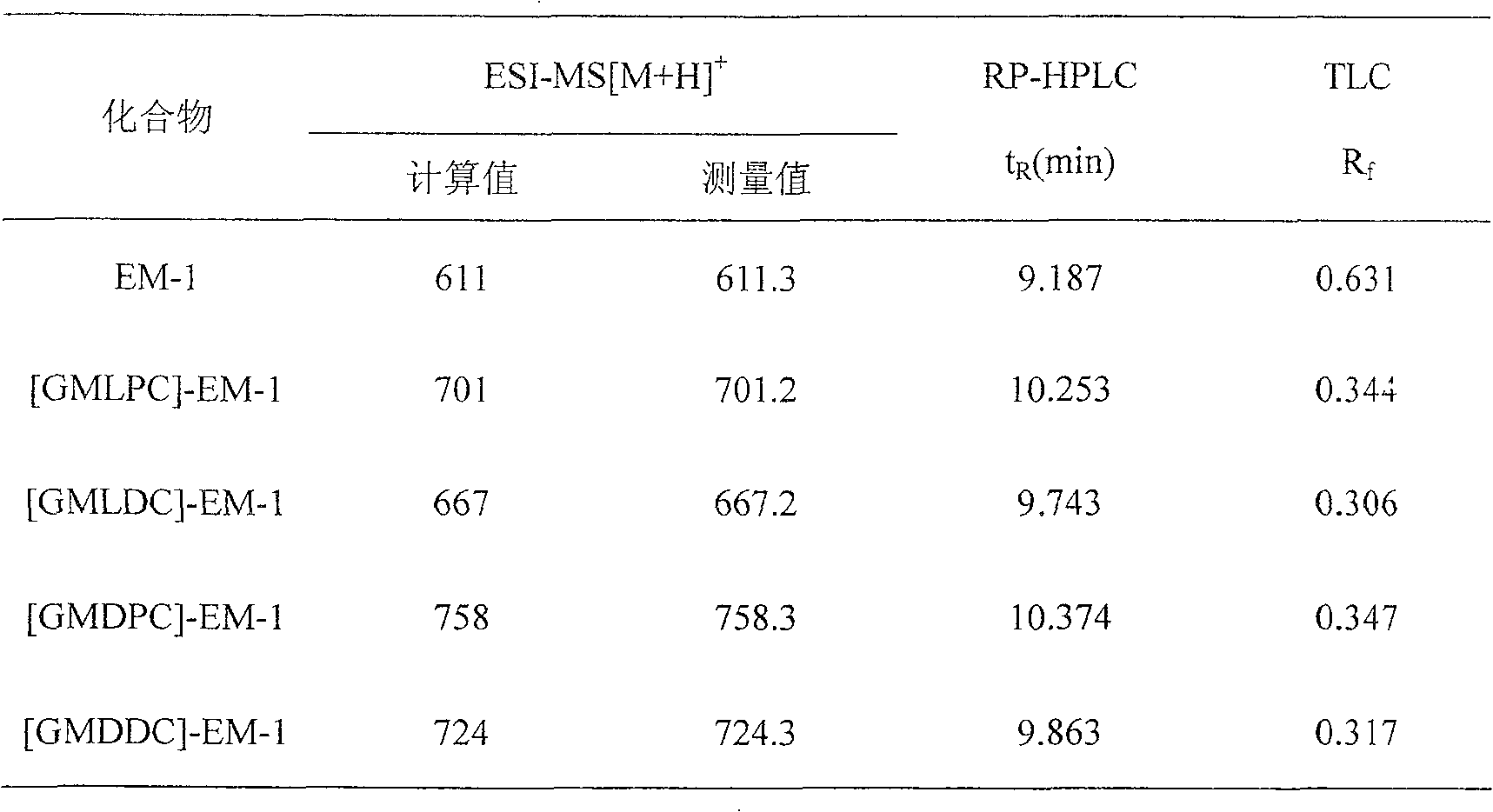
The present invention is four analogs of endomorphin-1, [GMLPC]-FM-1, [GMLDC]-EM-1, [GMDPC]-EM-1 and [GMDDC]-EM-1, prepared through liquid phase synthesis and their preparation process. Serial pharmacological activity identifying measurements shows that these four analogs have enzymolysis stability in brain plasmalemma and blood serum of mouse obviously higher than their precursor, longer pain relieving time, and higher integral liposolubility. The present invention is significant for developing endomorphin-1 into clinical polypeptide analgesic.
Description
technical field The present invention relates to a new class of analogs [GMLPC]-EM-1, [GMLDC]-EM-1, [GMDPC]-EM-1 and [GMDDC] of endomorphin-1 (Endomorphin-1, EM-1) ]-EM-1 and its preparation method. Background technique Endomorphin 1 (Tyr-Pro-Trp-Phe-NH 2 , EM-1) and endomorphin 2 (Tyr-Pro-Phe-Phe-NH 2 , EM-2) are two endogenous opioid peptides that selectively agonize mu opioid receptors with high efficiency. Studies have found that endomorphins can participate in the regulation of many functions such as pain perception, cardiovascular, respiratory, gastrointestinal, exercise, behavior, endocrine and immunity through binding to G protein-coupled μ opioid receptors, but its main role is Potent analgesic activity, can produce analgesic effect comparable to that of morphine with less side effects, especially they also show obvious analgesic activity in animal models of neuralgia. Because EM-1 has better therapeutic properties than EM-2, such as the separation of rewarding ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07K5/107C07K7/06A61K31/07A61K38/08A61P25/04
CPCY02P20/55
Inventor 王锐刘红美刘雪锋
Owner LANZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com