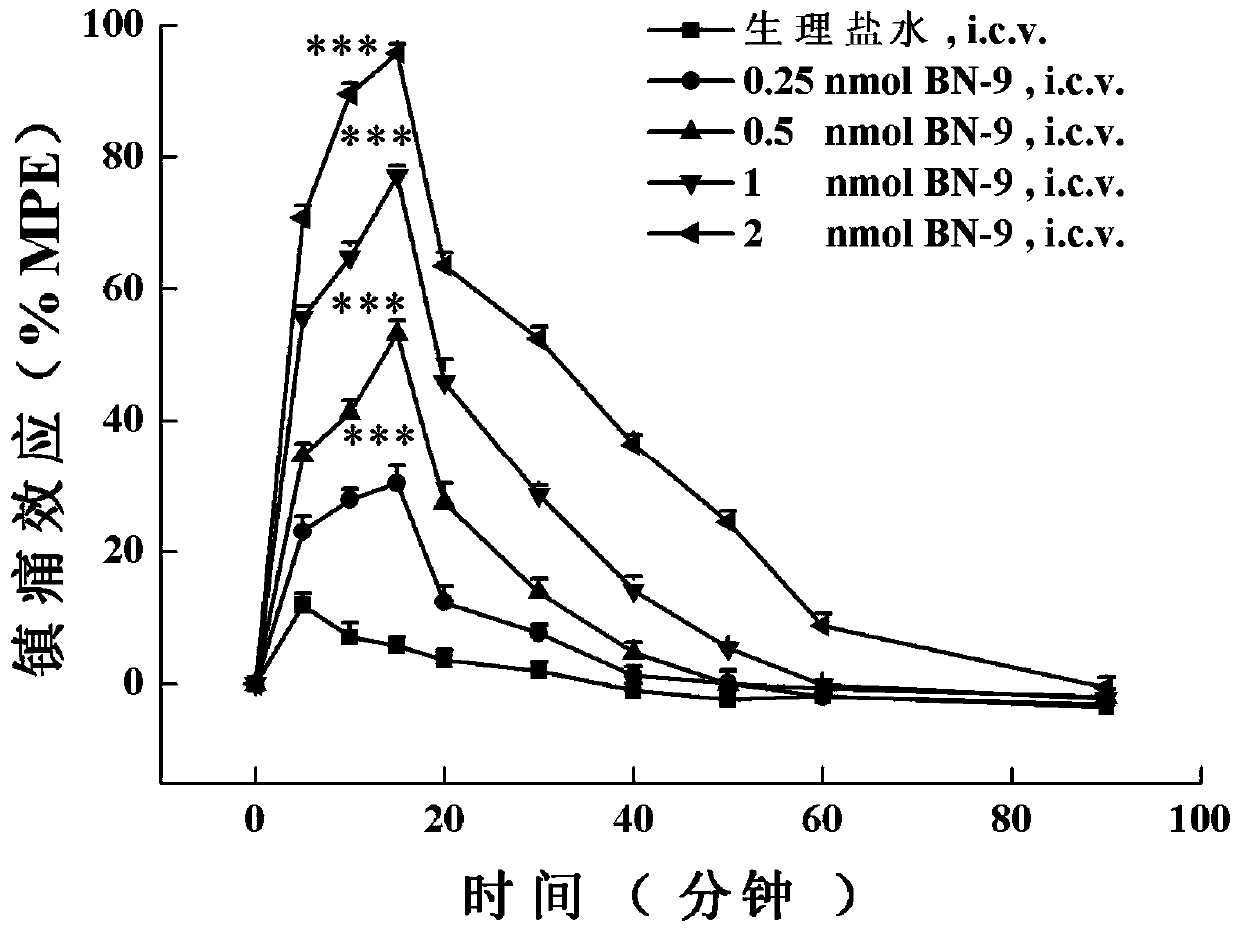
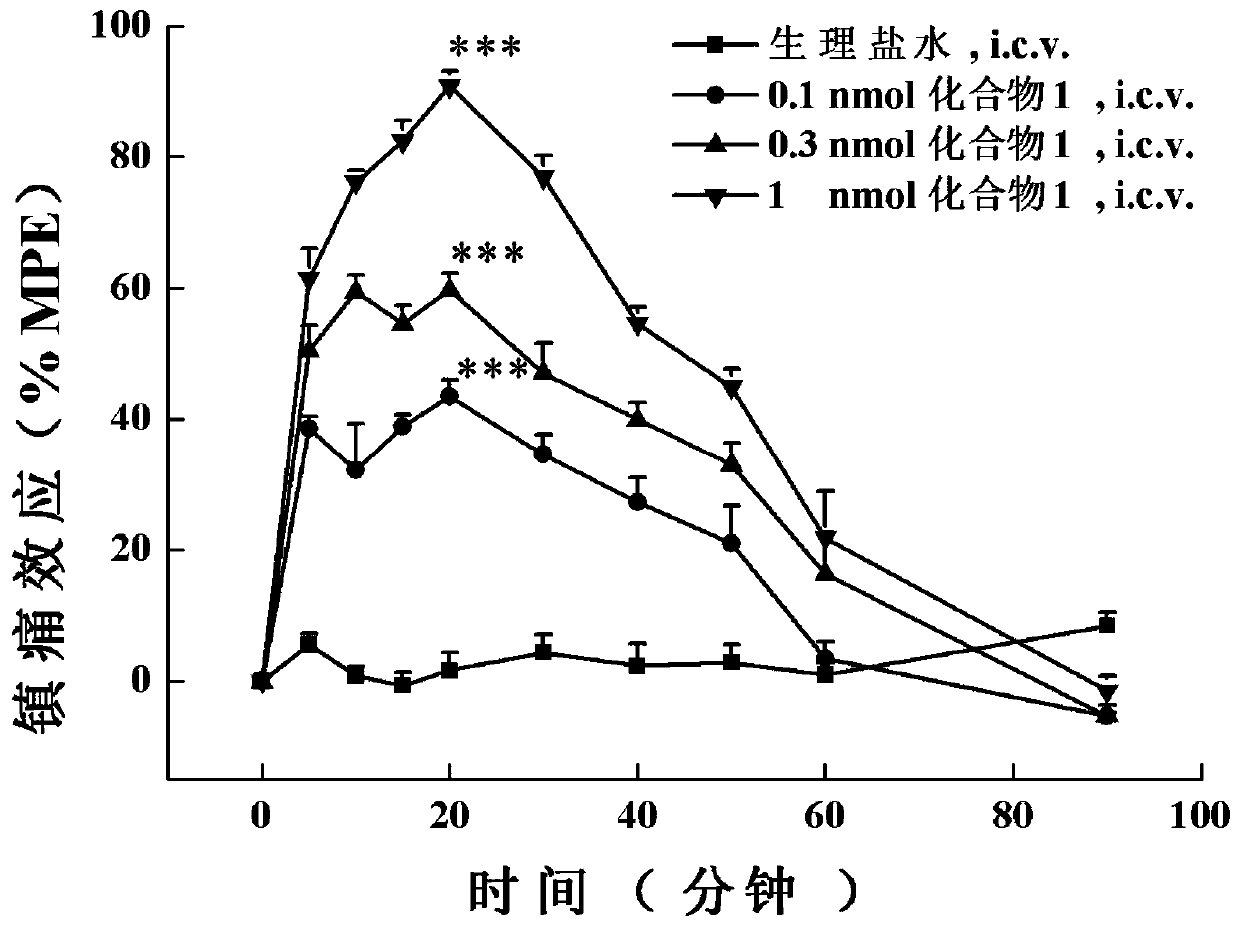
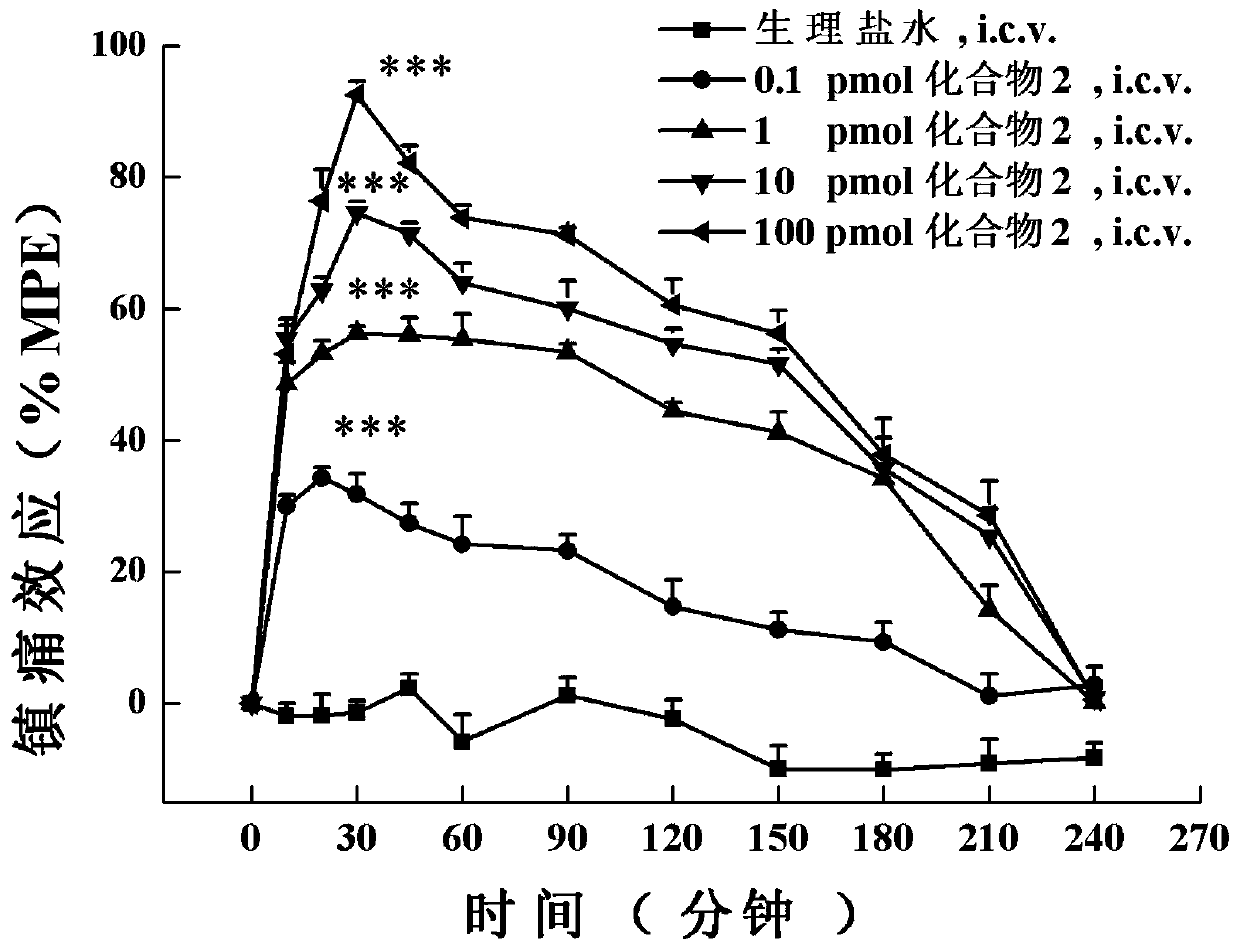
A class of analogs of opioid and neuropeptide ff receptor multi-target molecule bn-9 and its preparation method and application
A BN-9, neuropeptide technology, applied in the field of biochemistry, to achieve the effect of improving analgesic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The synthetic method of embodiment 1 compound 1-4:
[0063] Experimental reagent: resin is Rink-Amide-MBHA-Resin (substitution value S is 0.43mmol / g; Tianjin Nankai Hecheng Company), O-benzotriazole-N,N,N',N'-tetramethyl Urea-hexafluorophosphate (HBTU) (Shanghai Jier Biochemical Co., Ltd.), N-hydroxybenzotriazole (HOBt) (Shanghai Jier Biochemical Co., Ltd.), N,N-diisopropylethylamine (DIEA) ( Beijing Bailingwei), 1,8-diazabicycloundec-7-ene (DBU) (Beijing Bailingwei), ninhydrin is the product of Shanghai Reagent No. 3 Factory, dichloromethane (DCM), N,N-dichloromethane Methylformamide (DMF), hexahydropyridine (piperidine), methanol (MeOH) and pyridine were purchased from Tianjin No. 2 Reagent Factory, and trifluoroacetic acid (TFA), phenol (PhOH) and pyridine were all from Tianjin No. 1 Reagent Factory Products, and the above organic reagents are all re-distilled before use.
[0064] Experimental equipment: solid-phase peptide synthesizer (designed by our laboratory...
Embodiment 2
[0106] Example 2 The in vitro functional activity assay of compounds 1-4 to opioid and NPFF receptors:
[0107] In stable expression of Mu-, Delta-, Kappa-opioid and NPFF 1 and NPFF 2 In HEK293 cells of the receptor, the agonism of these five receptors was detected by examining the regulation of the multi-target cyclic peptide of the opioid and NPFF receptors on Forskolin-induced intracellular cyclic adenosine monophosphate (cAMP) accumulation active. The specific method is: stably expressing Mu-, Delta-, Kappa-opioid and NPFF 1 and NPFF 2 Recipient HEK293 cells were seeded in 24-well plates at 120,000 cells per well, and cultured in an incubator for more than 20 hours. At the beginning of the experiment, the cell culture medium was replaced with preheated serum-free medium containing 1 mM IBMX, and incubated at 37°C for 10 min. Then, 10 μl of the drug to be tested and 10 μM Forskolin (final concentration) were added to each well, and incubated at 37° C. for 30 minutes....
Embodiment 3
[0115] The in vivo analgesic activity assay of embodiment 3 compound 1-4:
[0116] The analgesic activity of these BN-9 analogues in vivo was detected by the photothermal tail-flick experiment of acute pain model mice by adopting two different levels of administration, central (lateral ventricle administration) and peripheral (subcutaneous administration).
[0117] Administration of the lateral ventricle at the central level requires prior implantation of the lateral ventricle to ensure the accuracy of the administration site and minimize the damage to the mice. The lateral ventricle implantation was carried out using a mouse stereotaxic instrument. Kunming male mice weighed 21±2 g. First, the mice were anesthetized by intraperitoneal injection of pentobarbital sodium (80 mg / kg), and all surgical instruments were prepared and sterilized. Then the hair of the operation area on the top of the mouse's head was clipped clean. Fix the head of the mouse on the stereotaxic apparat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com