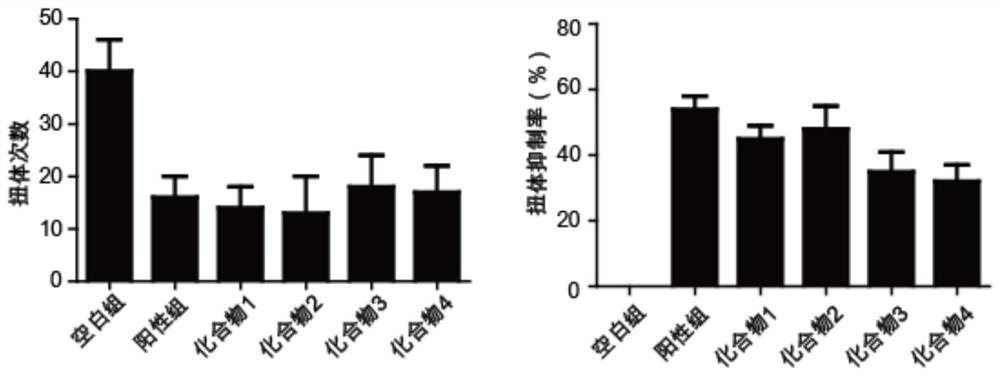
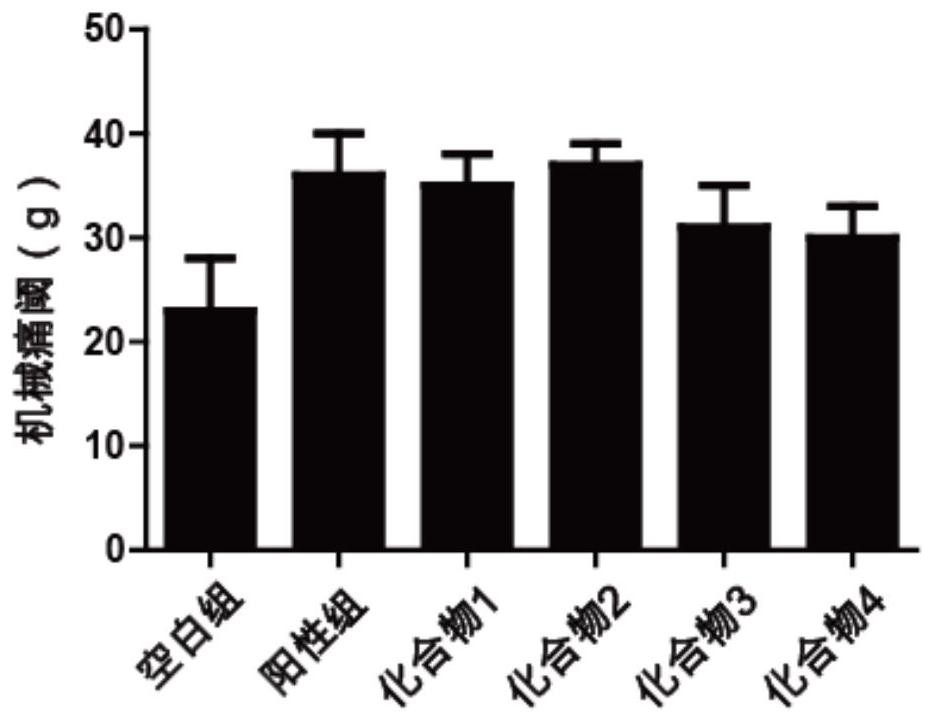
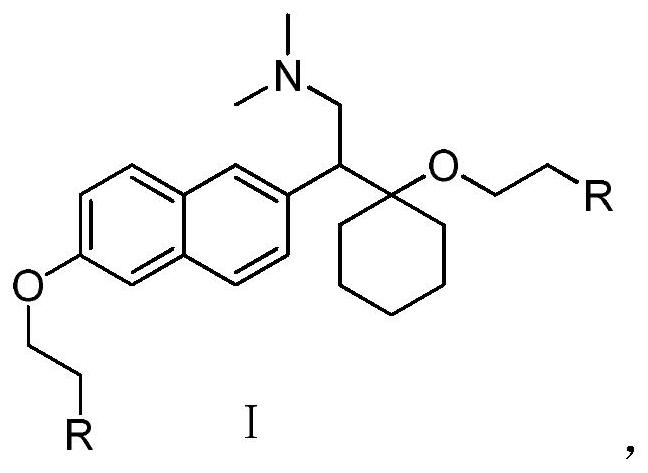
Naphthol derivative compound and application thereof in analgesia
A compound and naphthol technology, applied in the field of medicine, can solve the problems of low toxicity, high analgesic activity, good water solubility, etc., and achieve the effects of good development prospects, low toxicity, and high analgesic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the synthesis of compound II
[0027]
[0028] In the presence of a catalytic amount of N,N-dimethylformamide (0.5ml), 6-methoxy-2-naphthoic acid (10.0g, 49.45mmol) and thionyl chloride (45mL, 62.2mmol) were dissolved in dichloro The reaction was refluxed in methane (60ml) for 4 hours. After the reaction was complete, the system was added to a well-stirred solution of dimethylamine hydrochloride (10.1g, 123.87mmol) and triethylamine (17.4mL, 125.18mmol) in dichloromethane (50ml) while keeping the temperature below 5 ℃. After the addition was complete, stirring was continued at 5-10°C for 0.5 hours. Water (100.0 mL) was added to quench the reaction and the organic layer was separated. The aqueous layer was extracted with dichloromethane (3 x 50.0 mL), and the combined organic phases were washed with water (2 x 50.0 mL). The organic layer was dried over anhydrous sodium sulfate, and the organic layer was concentrated to obtain a crude product. To the...
Embodiment 2
[0039] Embodiment 2, the synthesis of compound 1
[0040]
[0041] At room temperature, 6-(2-(dimethylamino)-1-(1-hydroxycyclohexyl)ethyl)naphthalene-2-ol (7.73g, 24.66mmol) was gradually added to sodium hydride (1.8g, 75.00 mmol) in DMF (50 mL) suspension. The reaction mixture was stirred at room temperature for 1.5 hours, then 2-chloro-N,N-diethyl-1-amine (8.23 g, 60.67 mmol) was added to the system. Stirring at 100°C for 2 hours, after the completion of the reaction as detected by TLC, the reaction mixture was concentrated under reduced pressure, and the obtained crude product was dissolved in dichloromethane (200mL), extracted with 2mol / L HCl (2×50mL), and the aqueous phase Adjust to pH = 10 with 10% NaOH solution, then extract with dichloromethane (2 x 100 mL). The organic layer was dried over anhydrous sodium sulfate, and the solvent was concentrated under reduced pressure to obtain Compound 1 as an off-white solid, 10.74 g, with a yield of 85.1%. LC-MS (ESI, pos, ...
Embodiment 3
[0042] The synthesis of embodiment 3, compound 2
[0043]
[0044] At room temperature, 6-(2-(dimethylamino)-1-(1-hydroxycyclohexyl)ethyl)naphthalene-2-ol (8.00 g, 25.5 mmol) was gradually added to sodium hydride (1.8 g, 75.00 mmol) in DMF (50 mL) suspension. The reaction mixture was stirred at room temperature for 1.5 hours, then 1-(2-chloroethyl)piperidine (8.93 g, 60.5 mmol) was added to the system. Stirring at 100°C for 2 hours, after the completion of the reaction as detected by TLC, the reaction mixture was concentrated under reduced pressure, and the obtained crude product was dissolved in dichloromethane (200mL), extracted with 2mol / L HCl (2×50mL), and the aqueous phase Adjust to pH = 10 with 10% NaOH solution, then extract with dichloromethane (2 x 100 mL). The organic layer was dried over anhydrous sodium sulfate, and the solvent was concentrated under reduced pressure to obtain Compound 2 as an off-white solid, 11.56 g, with a yield of 84.6%. LC-MS (ESI, pos, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com