Pentamidine and death domain receptor ligand united application
A combined application, pentamidine technology, applied in the field of medicine, can solve the problem of enhancing the sensitivity of leukemia cells to induce apoptosis, and achieve the effect of treating leukemia and enhancing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation and cell culture of embodiment 1 pentamidine and TRAIL
[0022] Stock solutions of 1 mg / ml pentamidine from Sigma and 1 mg / ml TRAIL from PeproTech EC were prepared in ddH2O. K562 adopts conventional cell culture, RPMI-1640 culture solution containing 10% calf serum, 100u / ml of penicillin and streptomycin, at 37°C, saturated humidity, 5% CO 2 Cultured in an incubator, and the medium was changed every 3 to 4 days. Collect logarithmic growth phase cells, adjust the number of cells to 1×10 5 / ml, take 10mL to the culture dish, add pentamidine with the final concentration of 0.5, 1, 2, 5, 10 μg / ml respectively to act on K562 cells for 48 or 72 hours. The rest of the conditions are exactly the same. Incubate at 37°C and collect cells.
Embodiment 2
[0023] Embodiment 2 Pentamidine inhibits cell proliferation activity
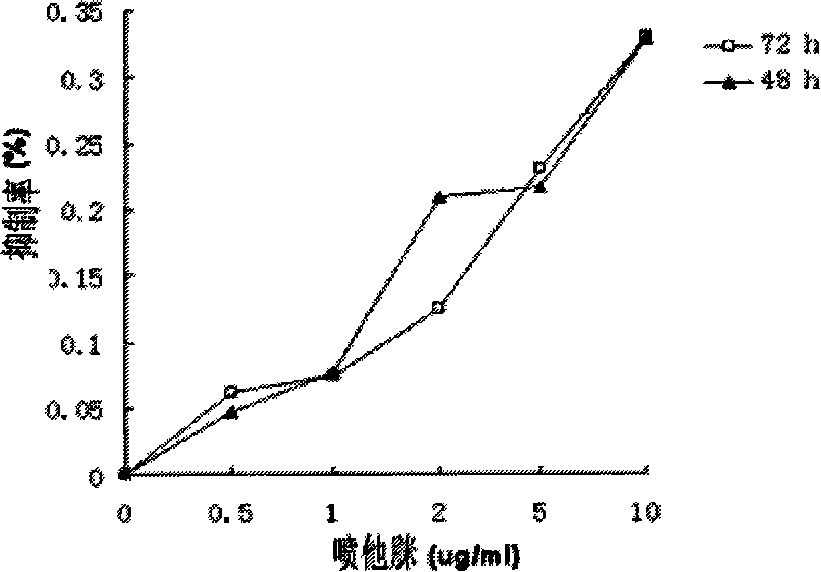
[0024] Tetramethylazolium salt (MTT) was purchased from Sigma. Prepare a 5 mg / ml stock solution with deionized water and store it at -20°C for later use. Take 100ul of each group of cells described in Example 1 and place them in a 96-well ELISA plate, then add 10ul of MTT storage solution to each well, incubate in a cell culture incubator at 37°C for 4 hours, take it out, add 100ul of isopropanol containing HCI, and mix The optical density (OD) was measured with a microplate reader with a wavelength of 570nM. The cell proliferation inhibition rate was calculated by (control OD-treatment OD) / control OD×%. Pentamidine has a certain inhibitory effect on cell proliferation at a concentration above 2ug / ml. For this reason, the range of 2-10 μg / ml pentamidine was used in combination with TRAIL.
Embodiment 3
[0025] Example 3 Pentamidine Enhances Sensitivity of Cells to TRAIL and Induces Apoptosis
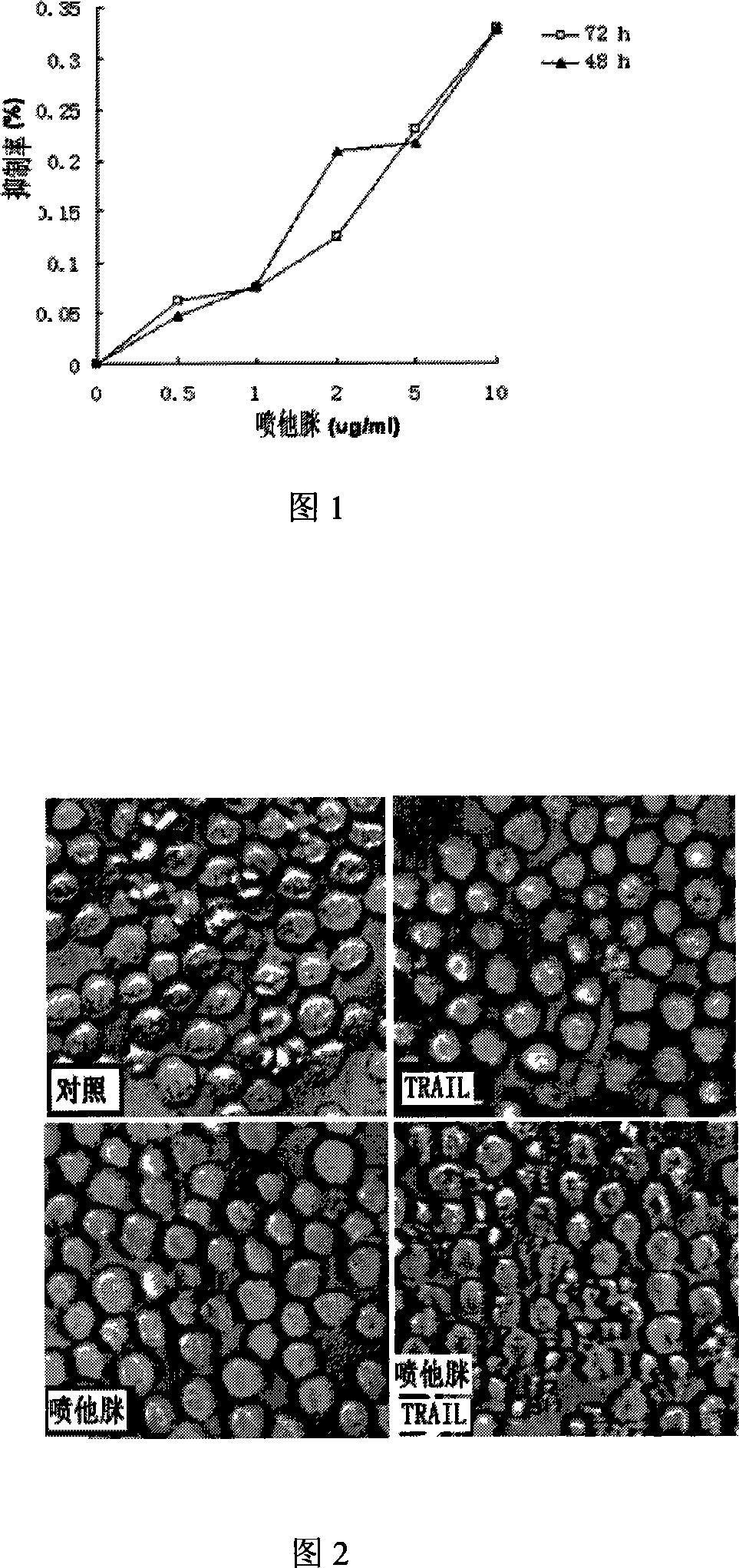
[0026] The cells were pretreated with 10 μg / ml pentamidine for 20 hours, followed by 200 ng / ml TRAIL for 4 hours. In addition, corresponding controls were set up, such as no drug, 10 μg / ml pentamidine for 24 hours, and 200 ng / ml TRAIL for 4 hours. Cell morphological changes were observed with a light microscope. There was no significant change in cells when pentamidine or TRAIL was used alone, but the morphology of cells appeared apoptosis characteristics after pretreatment with pentamidine.
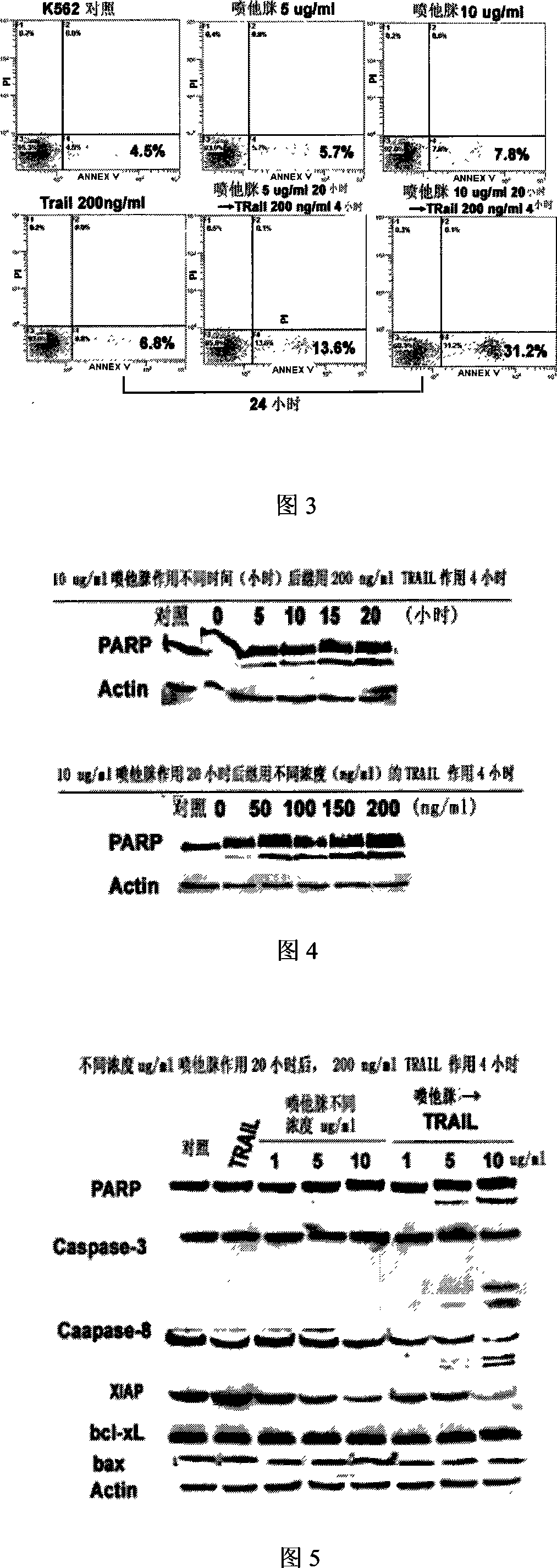
[0027] Preliminarily use 5, 10 μg / ml pentamidine to act on the cells for 20 hours, and then use 200ng / ml TRAIL for 4 hours, and set up corresponding controls. If no drug is added, 5, 10ug / ml pentamidine for 24 hours, 200ng / ml TRAIL for 4 hours The number of apoptotic cells was determined by Annexin V FITC / PI double-labeled apoptotic cells with flow cytometry (FACS) using the Annexin V apoptosis k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com