Materials and methods for treating and managing plaque disease
A vascular access and blood flow technology, applied in the field of enhancing vascular access and compositions, can solve problems such as prolonging functional dialysis access leaks, and achieve the effects of enhancing maturity and maintaining functional lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1: Human AV Fistula Study
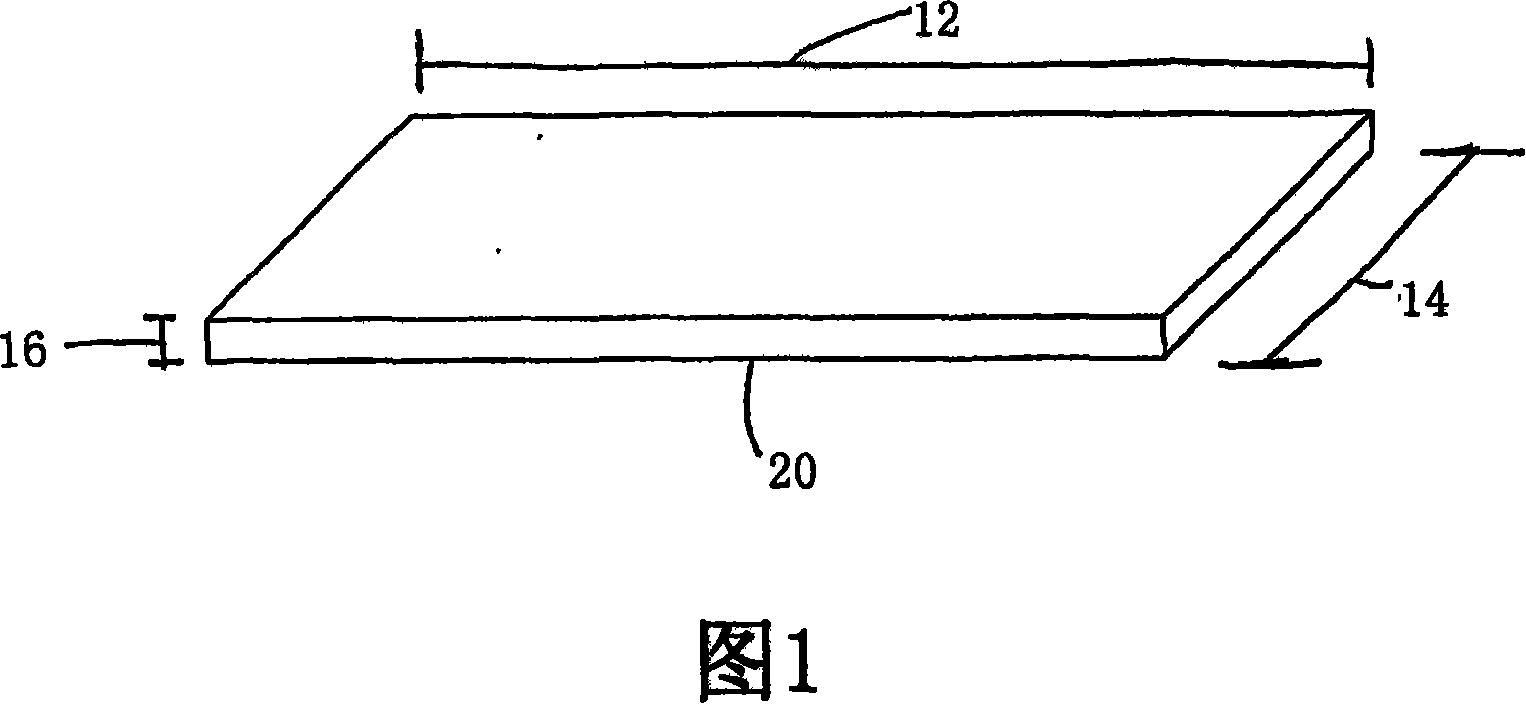
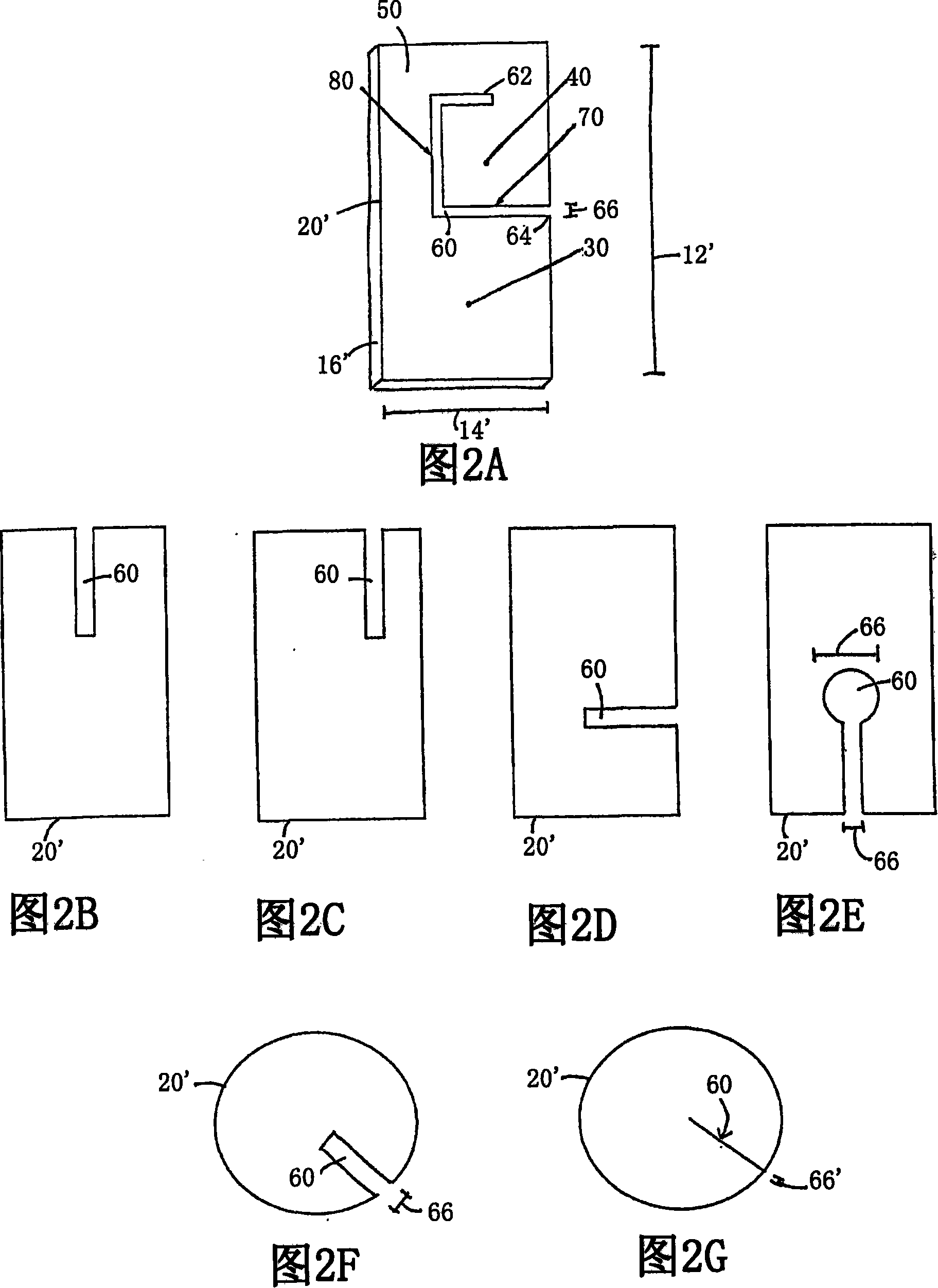
[0180] This example provides a protocol for testing and using preferred embodiments of implantable materials including vascular endothelial cells to enhance fistula maturation and / or prevent fistula maturation failure. Using standard surgical procedures, create an arteriovenous fistula at the desired anatomical location. The implantable material in flexible planar form is then deposited in the perivascular space adjacent to the surgically created fistula; details of one exemplary procedure are set forth below. As previously mentioned, the placement and configuration of the implantable material can be varied to suit the clinical situation. In this study, preferred exemplary flexible planar forms are shown at least in Figure 1 or 2A.
[0181] The following experiments and protocols provide sufficient guidance:
[0182] 1. Assess the failure of the arteriovenous fistula to mature at 3 months.
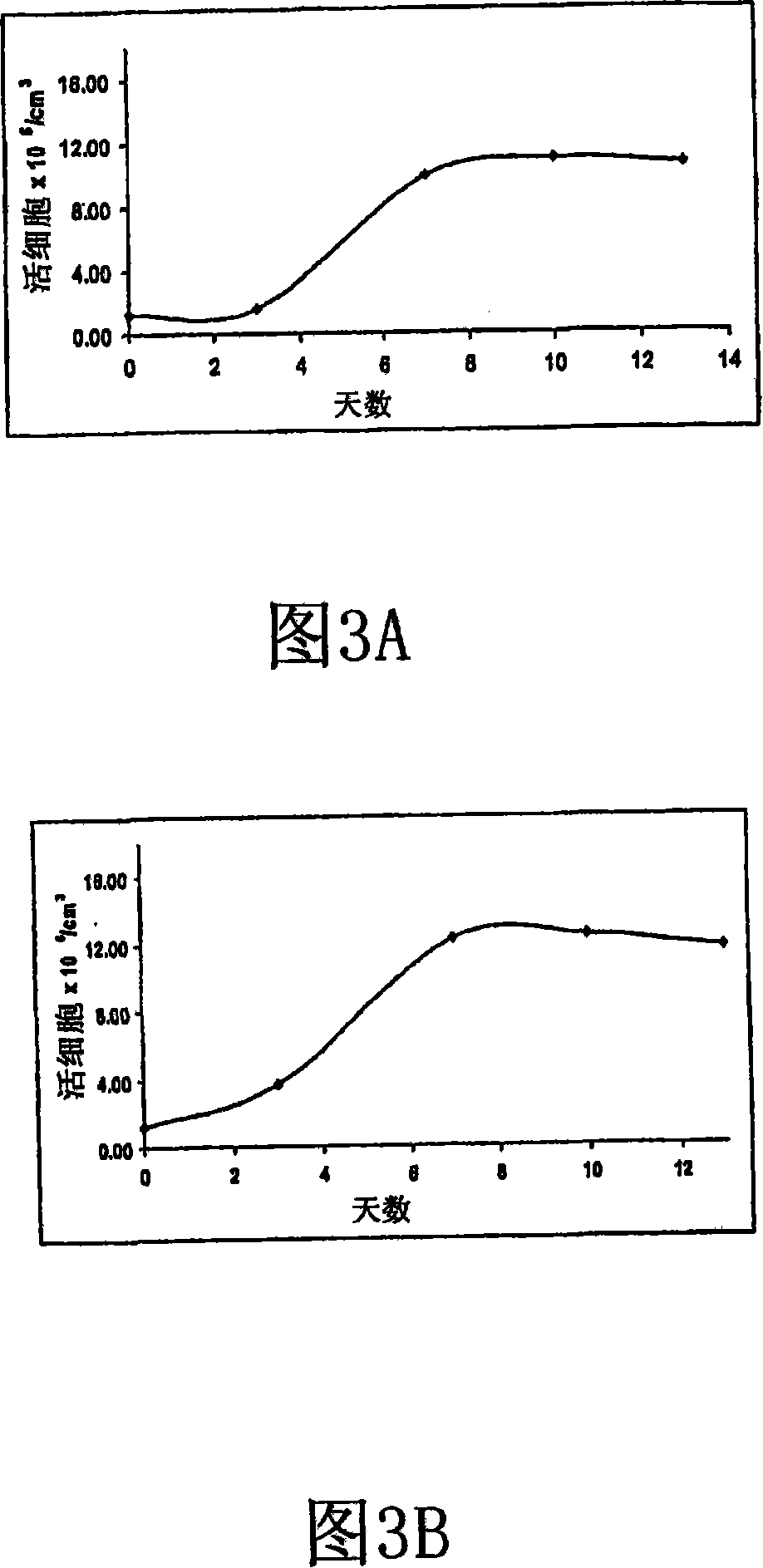
[0183] For this study, mature failure...
Embodiment 2
[0200] Example 2: AV Graft Animal Studies
[0201] This example provides a protocol for testing and using preferred embodiments of the invention to promote the formation of functional AV grafts in animal test subjects. Using standard surgical procedures, create an AV graft between the carotid artery and the jugular vein. The implantable material was then placed in the perivascular space adjacent to the anastomosis of each surgically created AV graft; details of an exemplary procedure are set forth below. As previously mentioned, the placement and configuration of the implantable material can vary. In this study, the implantable material in the form of a flexible planar surface is shown in Figures 4A, 4B and 4C.
[0202] Specifically, this study included 26 pig subjects who underwent AV transplantation. Conventional AV transplantation is performed according to standard operating techniques. Following completion of the graft procedure and establishment of blood flow throug...
Embodiment 3
[0226] Example 3: Clinical Study of Human AV Grafts
[0227] This example provides a protocol for testing and using the invention in human clinical subjects to promote the formation of functional AV grafts. Using standard surgical procedures, the AV graft anastomosis was fabricated at the desired anatomical location and an ePTFE prosthetic bridge was placed between the arterial and venous anastomosis. The implantable material was then deposited in the perivascular space adjacent to the anastomosis of each surgically created AV graft; details of an exemplary procedure are set forth below. As previously mentioned, the placement and configuration of implantable materials can be varied in a routine manner by the skilled practitioner.
[0228] Specifically, the study included human subjects who underwent AV transplantation. Traditional AV transplantation procedures are performed according to standard operating techniques. Following completion of the graft procedure and establi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com