Lactobionic azithromycin aqua compound, production and use of the same
A technology of azithromycin and lactobionic acid, applied in the field of medicine, to achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
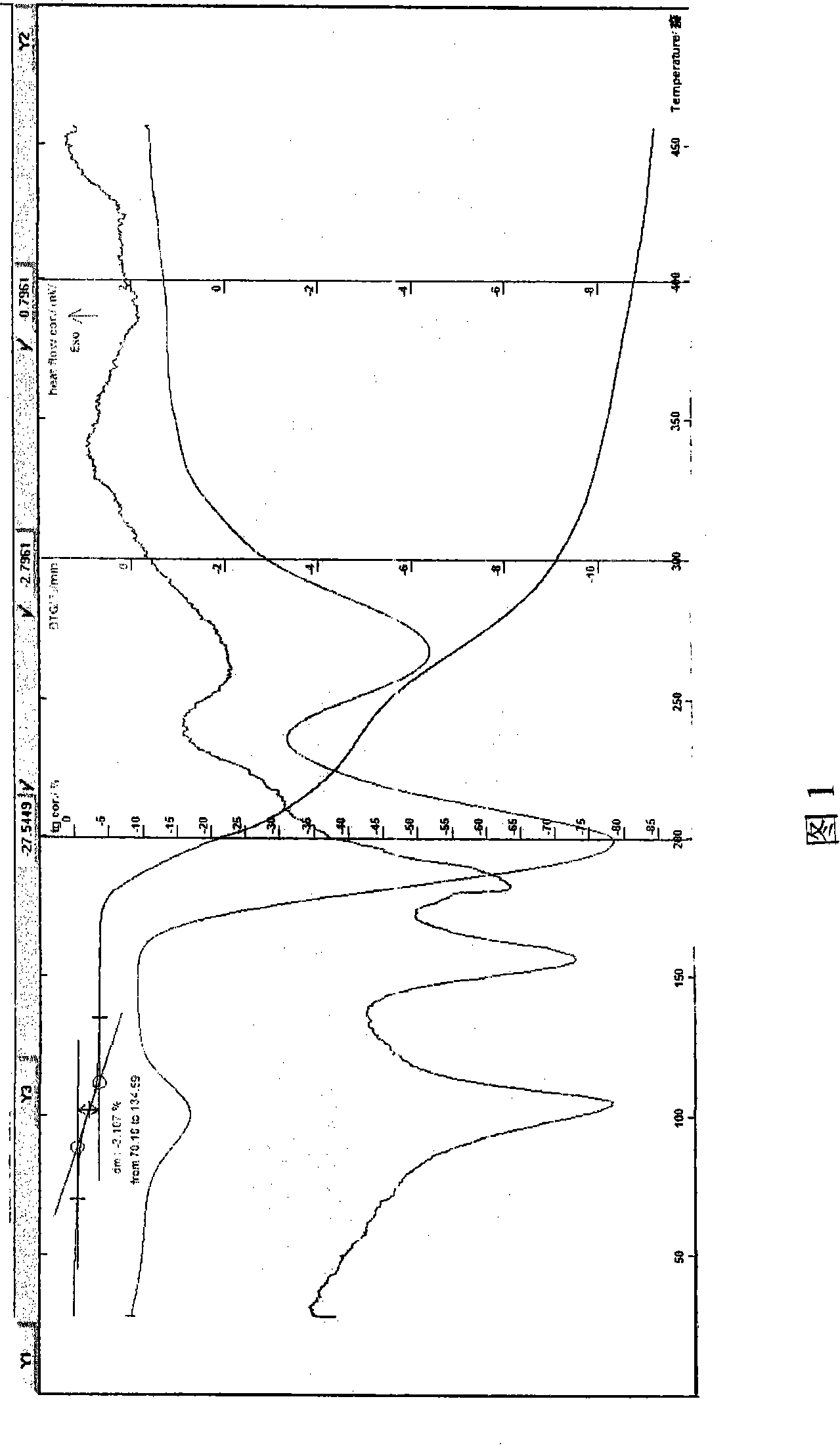
[0050] Example 1 In a three-necked flask, add ethanol acetone and azithromycin dihydrate, stir to dissolve, add lactobionic acid or its aqueous solution, stir continuously at 1-40°C to complete the reaction, slowly add ethyl formate, cool to 0-10 At about ℃, wait for the solid to precipitate, filter, rinse the solid with acetone, drain, and dry to obtain an off-white crystalline powder, easily soluble in water, HPLC: the retention time of the main peak of the sample is consistent with that of the reference substance of azithromycin, melting point: 157 -160°C, decomposed (uncorrected), Karl Fischer's method to determine the moisture is 3.03%, which is within the error range with the result that the sample contains 2.5 crystal waters (theoretical value 2.98%). TG-DTA: The weight loss at 60-130°C is about 3.108%, which is within the error range with the result that the sample contains 2.5 crystal waters. TG-DTA shows that there is an obvious endothermic peak before 130°C, indicati...
Embodiment 2
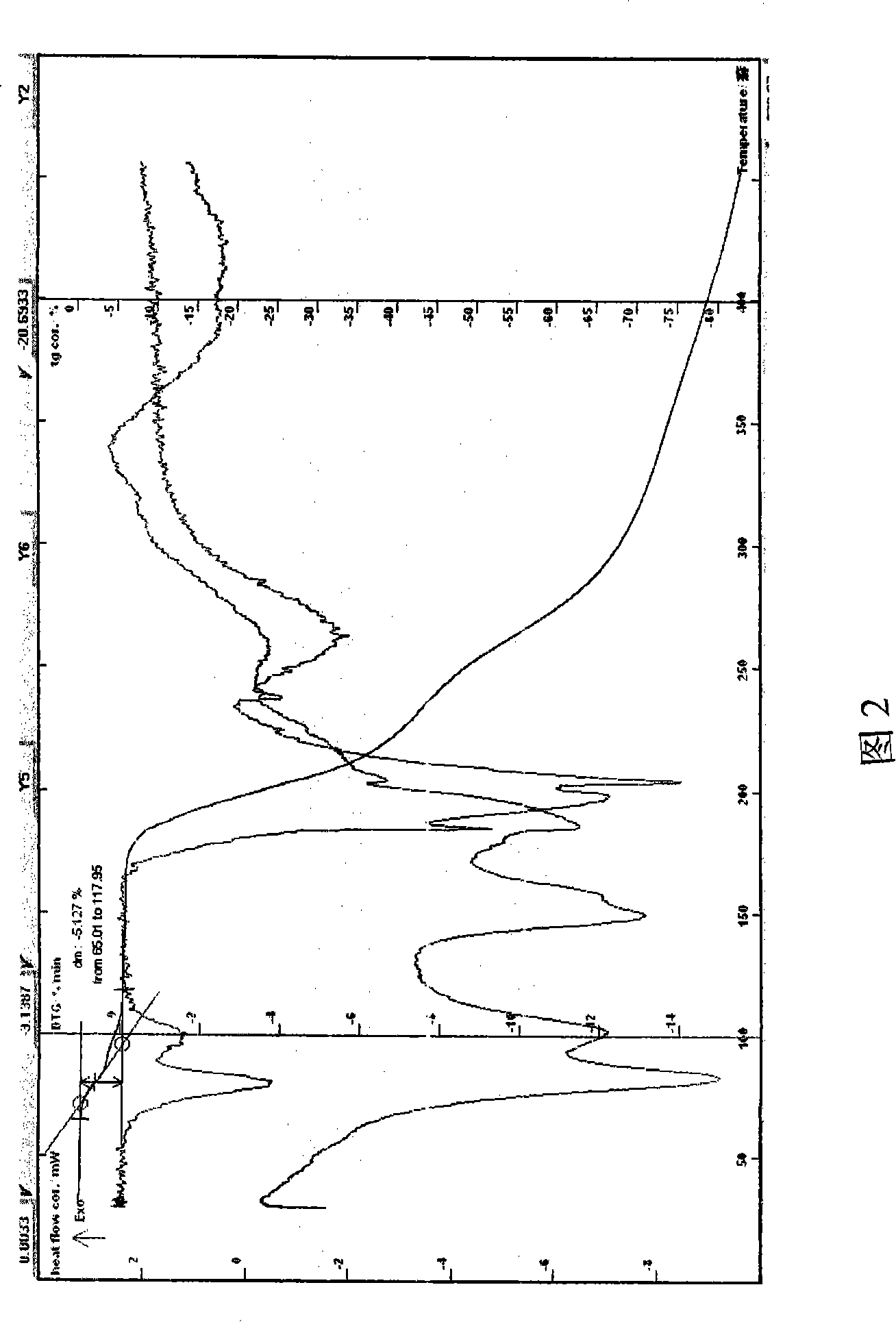
[0053] Example 2 In a three-necked flask, add ethanol to dissolve azithromycin, stir the lactobionic acid at 15-55°C to dissolve, and continue the reaction for 2 hours. After the reaction is completed, slowly add 2-15 times of ethyl formate and isopropanol (1:1), cooling, until the solid precipitates, filter, the solid is rinsed with methanol, drained, and dried at about 50°C to obtain off-white powder, easily soluble in water, HPLC: the retention of the main peak of the sample and the main peak of the azithromycin reference substance The time is consistent. Melting point: 151.5-156°C, decomposed, uncorrected; Moisture (Karl Fischer method): 5.25%, TG-DTA: 60-130°C weight loss of about 5.197%, which is the same as the result of the sample containing 4.5 crystal water (theoretical value %) within the margin of error. TG-DTA shows that there is an obvious endothermic peak before 130 ° C, indicating that the sample contains crystal water (see Figure 2). The obtained sample was ...
Embodiment 3
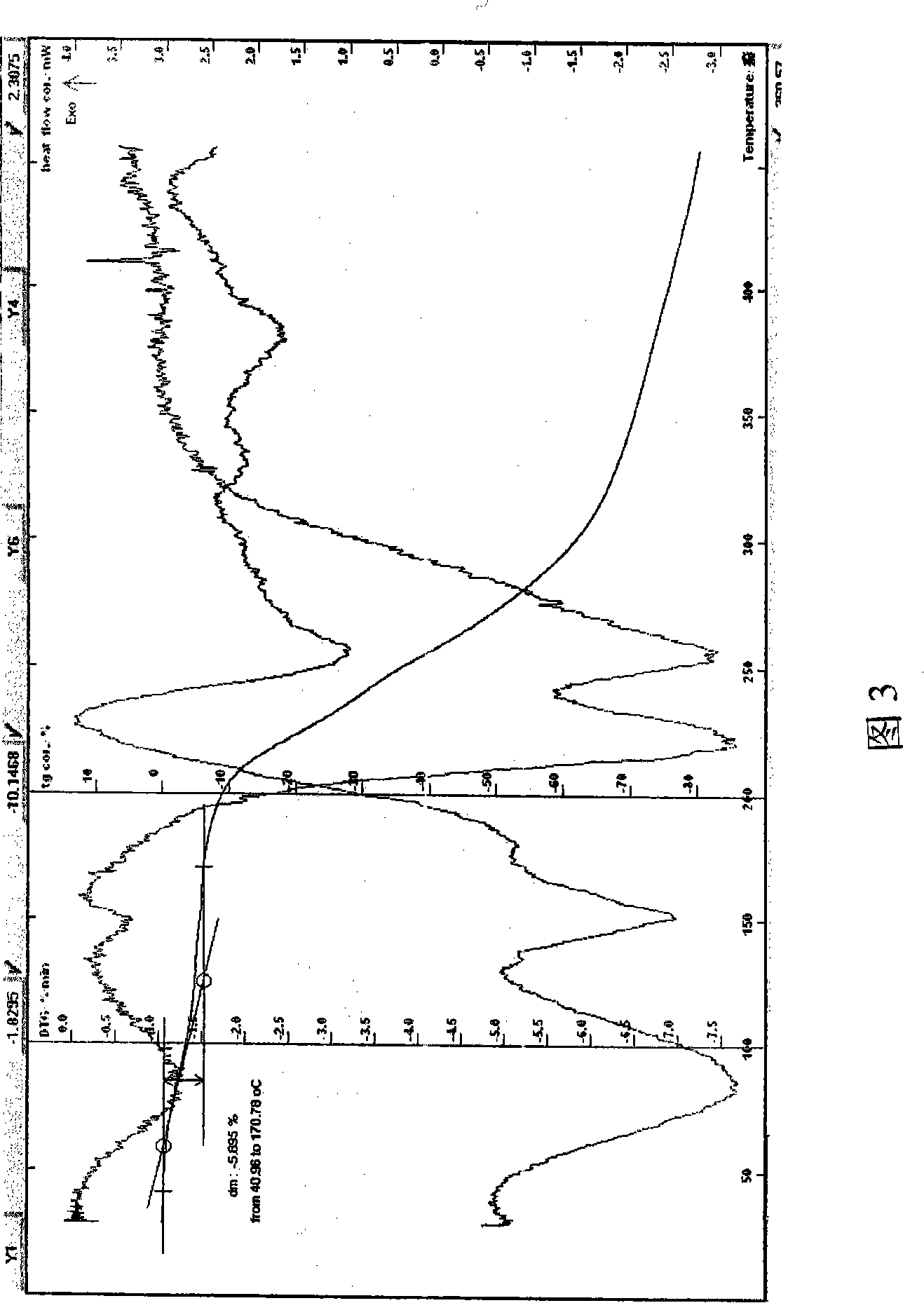
[0057] Example 3 Put lactobionic acid and azithromycin in a molar ratio (2:1) into a reaction flask, add water, heat, stir, control the temperature between 2-40°C, and react for 10-90min. After the reaction is completed, freeze it to - 50°C, keep it for 0.5-6 hours, lower the temperature of the condenser to -55°C, vacuumize, and gradually raise the temperature to about -20°C through the shelf, then keep it at about -20°C for about 24 hours, continue heating to heat up the medicine Keep at about 28°C for 6 hours to obtain a self-colored crystalline powder with a yield of 99%. The sample is easily soluble in water. HPLC: the retention time of the main peak of the sample is consistent with that of the azithromycin reference substance. Melting point: 153-157° C. (uncorrected), decomposed; Karl Fischer’s method determined moisture to be 6.05%, which was within the error range with the result that the sample contained 5 crystal waters (theoretical value 5.79%). TG-DTA: The weight lo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com