Optically active 3-methylcyclopentadecanone and method for producing intermediate thereof
一种甲基环十五烷酮、光学活性的技术,应用在光学活性3-甲基环十五烷酮及其中间体的制备领域,能够解决不能避免生成等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
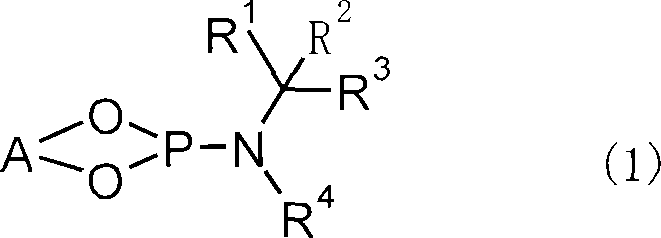
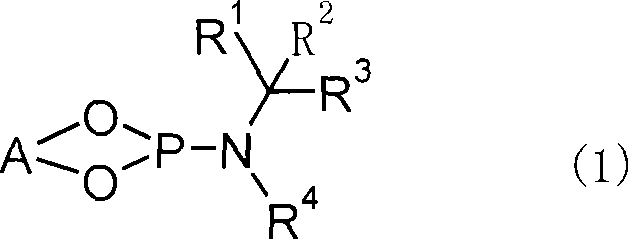
[0154] O,O'-(R)-(1,1'-binaphthyl-2,2'-diyl)-N-benzyl-N-(R)-(1-phenethyl)idene represented by the following chemical formula Synthesis of Phosphoramide (L-1)
[0155] [Chemical 12]
[0156]
[0157] Under nitrogen flow, in a 50 mL three-necked flask with a thermometer and a dropping funnel, add 20 mL of THF, 1.05 g (5.0 mmol) of (R)-(+)-N-benzyl-N-(1-phenethyl)amine The solution was cooled to -50°C, and 3.2 mL (5.0 mmol) of n-butyllithium (1.58 mol / L hexane solution) was added dropwise. After stirring at -50°C for 1 hour, 6.8 g (50 mmol) of phosphorus trichloride was added, the temperature was gradually raised to room temperature, and the mixture was stirred for 2 hours. Then, the solvent and phosphorus trichloride were removed under reduced pressure, and 20 mL of toluene was added to the residue. The toluene solution was cooled to -20°C, and 1.43 g (5.0 mmol) of (R)-(+)-1,1'-bi(2-naphthol), 2.53 g (25 mmol) of triethylamine, and 10 mL of toluene were added dropwise. mix...
Embodiment 2
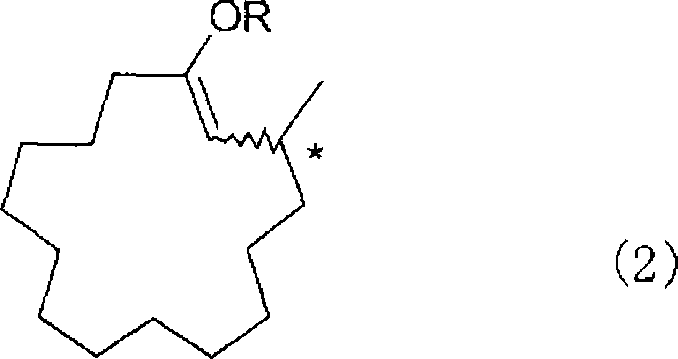
[0163] Synthesis of (R)-3-methyl-1-cyclopentadecenyl propionate represented by the following formula
[0164] [Chemical 13]
[0165]
[0166]Under nitrogen flow, into a 30 mL reaction flask with a thermometer, add 21.0 mg (0.04 mmol) of the optically active ligand O, O'-(R)-(1,1'-binaphthyl-2, obtained in Example 1, 2'-Diyl)-N-benzyl-N-(R)-(1-phenethyl)phosphoramidite, 7.2 mg (0.02 mmol) Cu(OTf) 2 and 7 mL of toluene, stirred at room temperature for 30 minutes. To this solution was added 1.4 mL (2.8 mmol) of a dimethylzinc toluene solution (2.0 mol / L), followed by stirring for 30 minutes. Then, the solution was cooled to -20°C, and 0.29 g (2.2 mmol) of propionic anhydride and 0.44 g (2.0 mmol) of 2-(E)-cyclopentadecenone were added dropwise. After completion of the dropwise addition, the mixture was stirred for 2 hours. Then, a 5% sulfuric acid aqueous solution was added to stop the reaction and separate liquids. The obtained organic layer was washed with water, and th...
Embodiment 3
[0174] Synthesis of (R)-Muskone represented by the following formula
[0175] [Chemical 14]
[0176]
[0177] To a 30 mL eggplant-shaped flask, 0.53 g (1.8 mmol) of (R)-3-methyl-1-cyclopentadecenyl propionate obtained in Example 2 and 20 g of toluene were added, and the mixture was stirred. After adding dropwise 0.39 g (2.0 mmol) of a 28% sodium methoxide-methanol solution at 20°C, the mixture was stirred for 1 hour. Then, after adding a 5% sulfuric acid aqueous solution to the reaction solution, the solution was separated, the organic layer was washed with water, and the solvent was removed under reduced pressure to obtain 0.60 g of a crude product. The crude product was purified by silica gel column chromatography to obtain (R)-muskone in a yield of 97%. The optical purity was determined by high performance liquid chromatography and the result was 86.1% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com