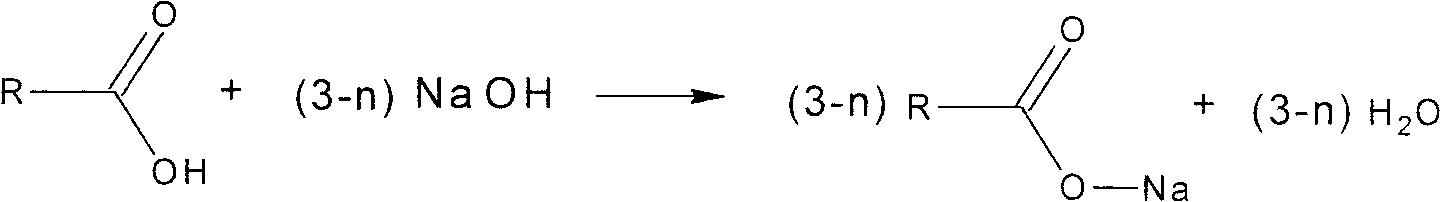
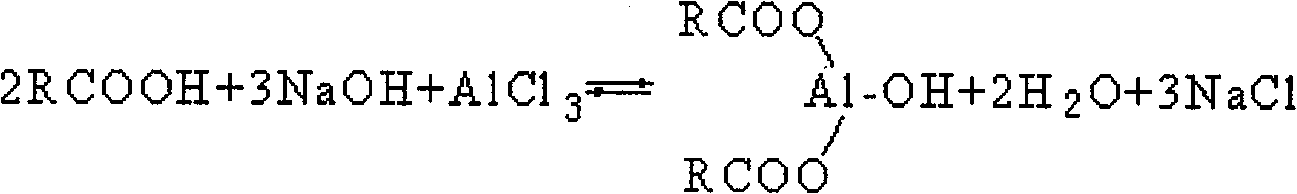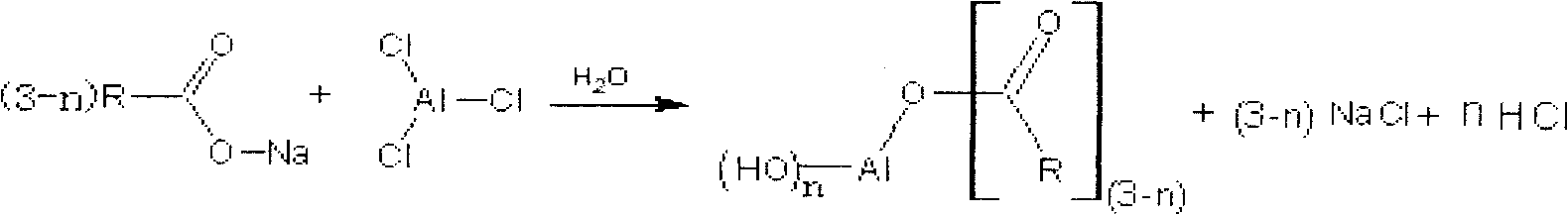Synthesis method for aluminum carboxylate nucleater without acidic byproduct
A technology of aluminum carboxylate and synthesis method, which is applied in the direction of carboxylate preparation, chemical instruments and methods, and compounds containing elements of group 3/13 of the periodic table, etc., to reduce requirements, reduce difficulty and cost, and improve purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take 180.00g (1 mole) of tert-butylbenzoic acid, slowly add 61.30g (1.5 moles) of 30wt% NaOH aqueous solution under stirring, and carry out neutralization reaction at 25°C. After reacting for 30min, the reaction system presents a clear homogeneous solution After that, 125.25g (0.5 moles) of 35wt% AlCl 3 ·6H 2 O aqueous solution was gradually added to the reaction system, the stirring speed was increased, and the reaction was carried out at 55° C. for 1.5 hr, the reaction was terminated, filtered, the filtrate was collected, and the pH value of the filtrate was measured; the soluble salt remaining in the nucleating agent was removed by water washing, dried, and calculated as The monohydroxyl salt is the synthetic reaction yield of the calculation basis, and the synthetic reaction yield is based on the reactant tert-butylbenzoic acid; the DSC method is used to measure the decomposition temperature; the analysis and determination is the nucleating agent purity based on the...
Embodiment 2
[0028] The operation steps are the same as in Example 1, except that the neutralization reaction temperature is 30° C., the reaction time is 1.0 hr, the amount of NaOH is 57.21 g (1.4 moles), the neutralization replacement / hydroxylation reaction temperature is 45° C., and the reaction time is 1.0 hr. hr. Analysis and measurement and application test are the same as in Example 1.
Embodiment 3
[0030] The operation steps are the same as in Example 1, except that the neutralization reaction temperature is 1° C., the reaction time is 1.0 hr, the amount of NaOH is 65.39 g (1.6 moles), the neutralization replacement / hydroxylation reaction temperature is 60° C., and the reaction time is 2.0 hr. hr. Analysis and measurement and application test are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com