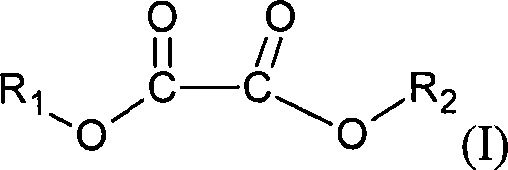
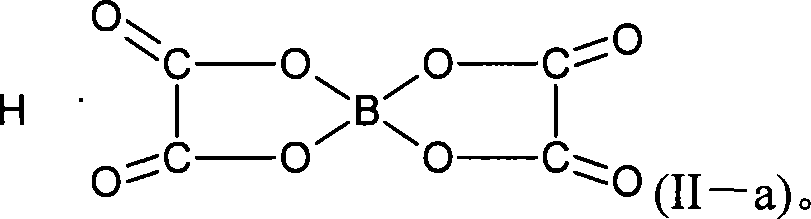
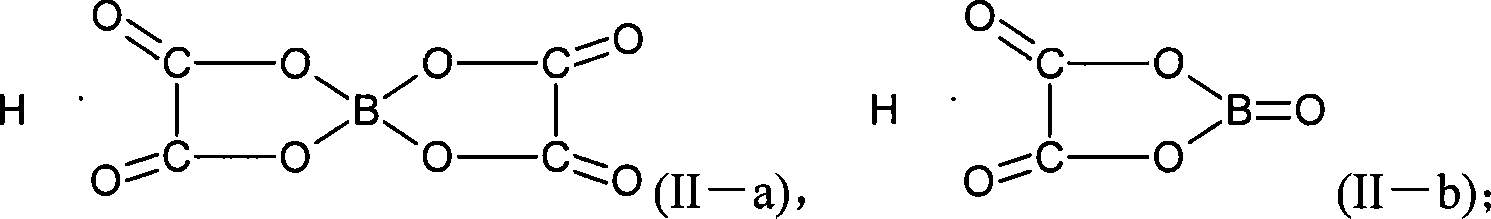Method for preparing Lithium bis(oxalate)borate
A lithium oxalate borate and lithium bisoxalate borate technology, which is applied in the field of preparing high-purity lithium oxalate borate, can solve the problems of cumbersome steps, affecting product purity, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095]In a three-necked flask containing 157.3 g (1.334 mol) of methyl oxalate, 30 g (0.4854 mol) of boric acid, and 22 g (0.524 mol) of lithium hydroxide, the temperature was raised to 110° C. under stirring, and the generated methanol was extracted. The temperature is gradually increased to 250°C until there is almost no extraction; the vacuum is turned on to keep the system vacuum at -100Pa, dried for 8-12 hours, and cooled to obtain a solid powder. Use 1000g of acetonitrile as a solvent to feed the reaction product, stir at room temperature for 1 to 2 hours, and filter to obtain a filter cake. After analyzing the components of the filter cake with infrared spectroscopy, it is known that the filter cake is lithium monooxalate borate. Carry out rectification under reduced pressure on the filtrate obtained above, recover 30~60℃ / 2~5kPa distillate 843g, the still material after concentration, filter, obtain filter cake, dry 60℃ / 80Pa, 71g; Through infrared spectrum analysis, γ: ...
Embodiment 2
[0097] In a three-neck flask containing 194.8 g (1.334 mol) of ethyl oxalate, 30 g (0.4854 mol) of boric acid, and 22 g (0.524 mol) of lithium hydroxide, the temperature was raised to 110° C. under stirring, and the generated ethanol was extracted. The temperature is gradually increased to 250°C until there is almost no extraction; the vacuum is turned on to keep the system vacuum at -100Pa, dried for 8-12 hours, and cooled to obtain a solid powder. Use 1000g of acetonitrile as a solvent to feed the reaction product, stir at room temperature for 1 to 2 hours, and filter to obtain a filter cake. After analyzing the components of the filter cake with infrared spectroscopy, it is known that the filter cake is lithium monooxalate borate. Carry out rectification under reduced pressure on the filtrate obtained above, recover 30~60℃ / 2~5kPa distillate 853g, filter the still material after concentration, obtain filter cake, dry 60℃ / 80Pa, 73g; Analyze by infrared spectrum, γ: 1818, 1781...
Embodiment 3
[0099] In a three-necked flask containing 269.5 g (1.334 mol) of butyl oxalate, 17 g (0.2427 mol) of boron oxide, and 22 g (0.524 mol) of lithium hydroxide, the temperature was raised to 110° C. under stirring. The temperature was gradually raised to 250°C, and the produced butanol was extracted until almost no extraction; the vacuum was turned on, and the vacuum of the system was kept at -100Pa, dried for 8-12 hours, and cooled to obtain a solid powder. Use 1000g of acetonitrile as a solvent to feed the reaction product, stir at room temperature for 1 to 2 hours, and filter to obtain a filter cake. After analyzing the components of the filter cake with infrared spectroscopy, it is known that the filter cake is lithium monooxalate borate. Carry out rectification under reduced pressure on the filtrate obtained above, recover 30~60℃ / 2~5kPa distillate 813g, filter the still material after concentration, obtain filter cake, dry 60℃ / 80Pa, 69g; Analyze by infrared spectrum, γ: 1818,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap