Pharmaceutical combination comprising an antibacterial agent and an active substance selected from carveol, thymol, eugenol, borneol and carvacrol
A technology of thymol and therapeutic active substances, applied in the field of pharmaceutical compositions containing antibacterial drugs and active substances selected from carveol, thymol, eugenol, borneol, carvacrol, capable of solving shortening, immune Inhibition of difficult-to-treat issues such as life expectancy for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0056] Example 1: Treatment of different strain
[0057] In vitro test: determination of the minimum bactericidal concentration (MBC) against different strains
[0058] Experiments were performed with several Gram-negative and Gram-positive strains, which were isolated in a hospital setting and had different sensitivities. The antibiotic used is amoxicillin, which is one of the most effective and widely used antibiotics.
[0059] The antibacterial pharmaceutical composition according to the present invention is prepared by mixing different concentrations of amoxicillin and carveol at a sub-inhibitory concentration of 0.3 g / l solution or excipient (equivalent to 0.3 mg / ml). The lowest concentration of amoxicillin to produce a bactericidal effect in combination with 0.3 mg / ml carveol was determined. In each case, antibiotic activity was determined with amoxicillin alone, or with carveol alone, or with a composition of the invention.
[0060] Table 1 gives the results of th...
example 2
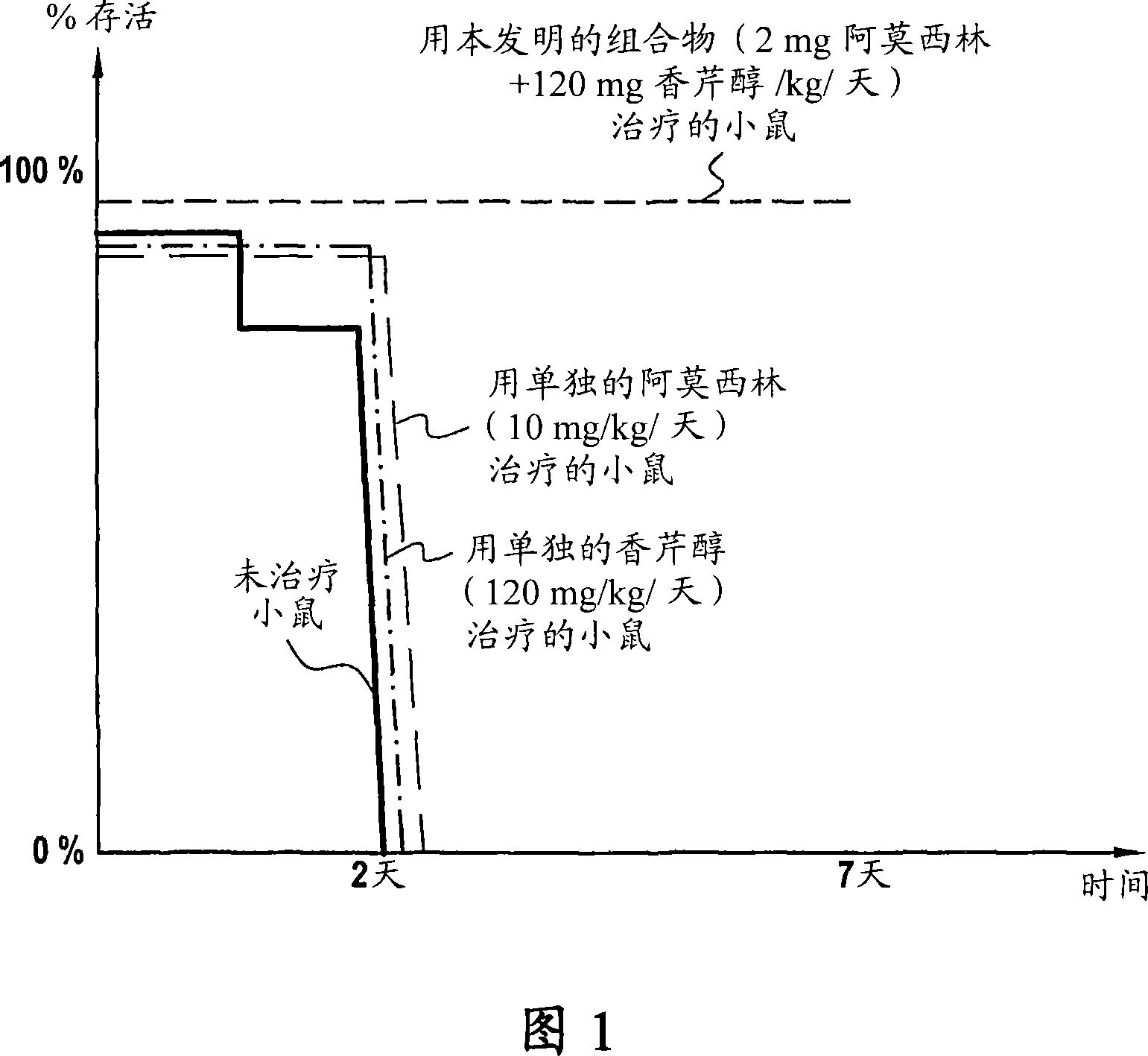
[0066] in vivo test
[0067] Groups of 10 mice were experimentally infected by intraperitoneal injection of 1,000,000 cells (colony forming units) of amoxicillin-resistant Klebsiella pneumoniae.
[0068] The first group consisted of control mice, which were infected and not treated.
[0069] The second group consisted of infected mice which were treated by gavage with amoxicillin alone at a dose of 10 mg / kg body weight / day 24 hours after their infection.
[0070] The third group consisted of infected mice which were treated with carveol alone at a dose of 120 mg / kg body weight / day by means of gavage 24 hours after their infection.
[0071] The fourth group consisted of infected mice, 24 hours after their infection, with the aid of gavage and with a dose of 2 mg / kg body weight / day of amoxicillin and 120 mg / kg body weight / day of carveol with the drug of the invention Infected mice were treated with the composition (AMOX-P).
[0072] Survival was measured over time. The res...
example 3
[0079] Example 3: Treatment of different strains with carveol-enhanced amoxicillin (Ampi-P)
[0080] Experiments were performed with several drug-resistant strains isolated in hospital settings. The antibiotic used was ampicillin from the beta-lactam class, which is one of the most widely used antibiotics. The antibiotic pharmaceutical composition according to the present invention is prepared by mixing different concentrations of ampicillin and sub-inhibitory concentrations of carveol in 0.3 g / l solution or excipient. This pharmaceutical composition of the present invention is called Ampi-P, standing for enhanced ampicillin. In each case, antibiotic activity was determined with ampicillin alone, or with carveol alone, or with a composition of the invention.
[0081] Table 2 presents the results of the static tests used to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) in μg / ml.
[0082] Table 2
[0083] Bacteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com