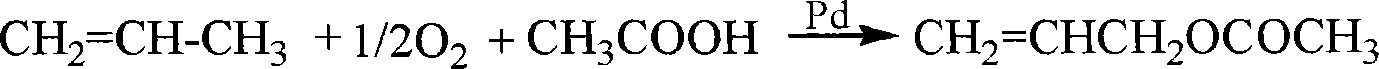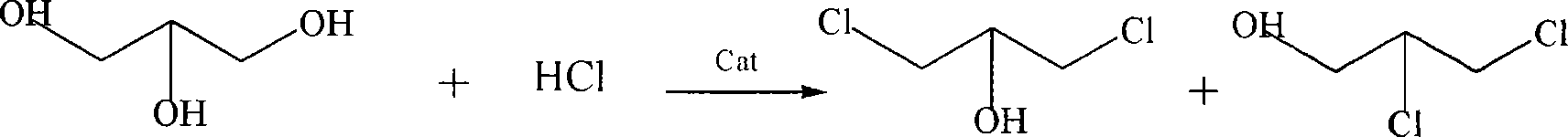Method for preparing dichloropropanol by glycerol
A technology of dichloropropanol and glycerin, which is applied in the introduction of halogen preparation, organic chemistry, etc., can solve the problems of low equipment utilization, high process energy consumption, and low reaction rate, and achieve increased reaction rate, high content, and glycerin conversion high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In the jet reactor, add 986g of industrial glycerol (91.9% by weight, 9.84mol) and 98g of adipic acid, the amount of catalyst is 9.9%, heat to 110°C, feed HCl gas to react, first pass 7.7mol HCl / h 2.05h, and then 3.18h at 3.3molHCl / h, a total of 24.4molHCl was introduced, and the molar ratio of glycerol to HCl gas was 1:2.5. During the reaction, the temperature was controlled at 115°C. The aeration time of hydrogen chloride was 5.23h, and 764g of chlorinated liquid was obtained, and 1134g of distillate was obtained by cooling during the reaction. The composition of the reaction product is:
[0032] Material name
Embodiment 2
[0034] Then embodiment 1 repeats test continuously 3 times, and condition is all the same with the condition of embodiment 1, does not emit at the bottom of the still, just adds industrial glycerin (91.9% by weight), and adds adipic acid and hydrogen chloride gas. The amount added is shown in the table below:
[0035] times
Industrial glycerin (g)
Adipic acid (g)
the first time
1412
20
1445
the second time
845
0
770
the third time
1010
20
925
[0036] The composition of the reaction product is:
[0037] times
Embodiment 3
[0039] In the jet reactor, add 1200g industrial glycerin (91.9% by weight, 12.0mol) and 57.5ml (60.4g) of acetic acid, the amount of catalyst is 5.5%, heat to 110 ℃, pass into HCl gas to react, first with 3.3mol HCl / h was fed for 3 h, and then 1.7 mol HCl / h was fed for 5.9 h, a total of 19.8 mol of HCl gas was fed, and the molar ratio of glycerol to HCl gas was 1:1.6. During the reaction, the temperature was controlled at 115°C. The aeration time of hydrogen chloride was 8.9h, and 882g of chlorinated liquid was obtained, and 1102g of distillate was obtained by cooling during the reaction.
[0040] The composition of the reaction product is:
[0041] Material name
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com