Method for production of a bioengineered form of tissue plasminogen activator
A technology of plasminogen and activator, applied in biochemical equipment and methods, drug combinations, enzymes, etc., can solve the problem of low incidence of non-cerebral hemorrhage events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The DNA sequence encoding tissue plasminogen activator was obtained by de novo synthesis. This approach can be more conducive to better codon optimization for a particular cell line. And, making synthetic DNA the subject of eukaryotic / prokaryotic expression provides isolatable quantities of polypeptides exhibiting the biological properties of naturally occurring t-PA and the biological activities of t-PA in vivo and in vitro.
[0029] The nucleotide sequence (TENECT1) encoding recombinant tissue plasminogen activator is shown in SEQID.No.1. Codons in the DNA sequence encoding tissue plasminogen activator have been altered as part of a codon optimization process to ensure optimized expression of the recombinant protein in mammalian cell lines such as CHO K1 and HEK293, these altered Codons for are highlighted with capital letters. SEQ ID.No.2 represents the codon-optimized nucleotide sequence (TENECT2) encoding tissue-type plasminogen activator
[0030] The paired seq...
Embodiment 2
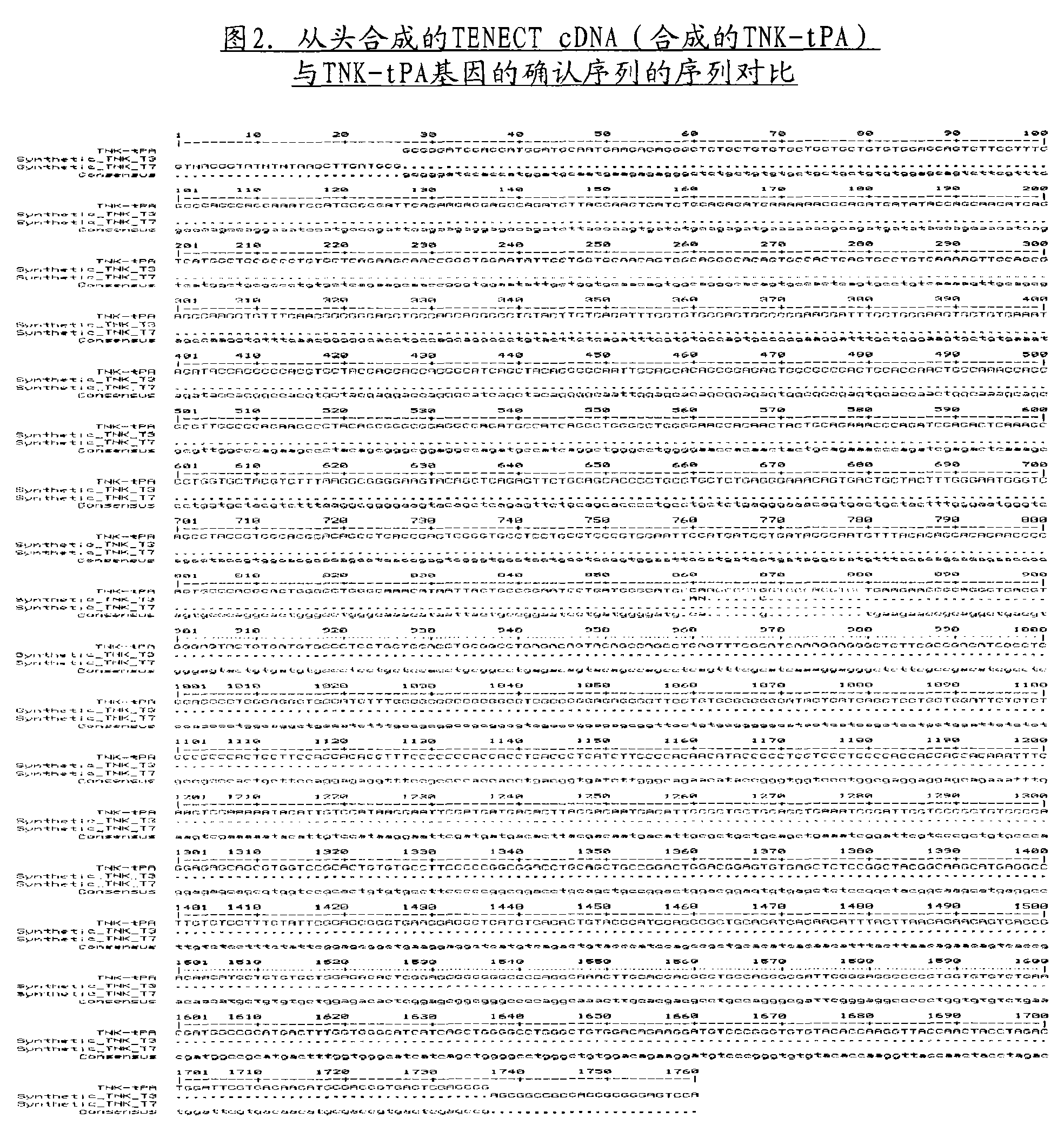
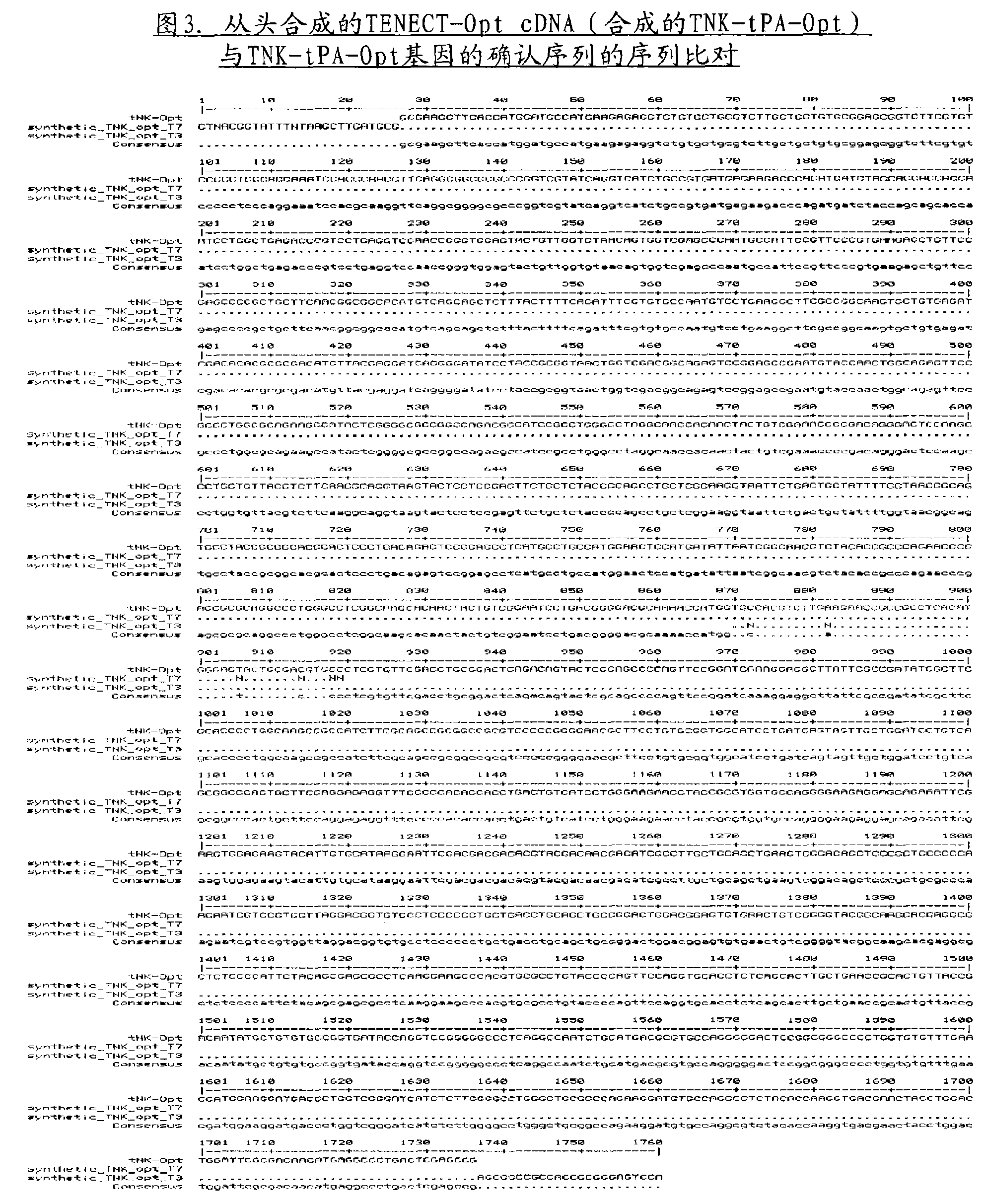
[0031] Example 2: Confirmation of the authenticity of the de novo synthesized cDNA encoding tissue-type plasminogen activator
[0032] The authenticity of the de novo synthesized cDNA provided by a commercial service provider was confirmed by automated DNA sequencing, and the results obtained are depicted in Figures 2 and 3.
Embodiment 3
[0033] Example 3: Subcloning TENECT and TENECT-Opt cDNAs into pcDNA3.1D / V5-His mammalian cell-specific expression vector
[0034] After verifying the authenticity of the de novo synthesized cDNA molecules (TENECT and TENECT-Opt) by automated DNA sequencing as shown above, TENECT and TENECT-Opt were respectively subcloned into the mammalian cell-specific expression vector pcDNA3.1D / V5-His , to generate constructs for transfection. The methodology employed is detailed below:
[0035] A. Reagents and Enzymes
[0036] 1. QIAGEN Gel Extraction Kit and PCR Purification Kit
[0037] 2. pcDNA 3.1D / V5-His vector DNA (Invitrogen)
[0038] enzyme
supplier
U / μl
10× buffer
1. BamHI
Bangalore Genei
10
Buffer E
2. Xhol
Bangalore Genei
10
Buffer E
3. Hind III
Bangalore Genei
20
Buffer E
4. Xhol
Bangalore Genei
10
Buffer E
5. T4 DNA ligase
Bangalore Genei
40
Ligase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com