Antiproliferative drug
A technology of hyaluronic acid and conjugates, which is applied in the field of esterified conjugates of hyaluronic acid, and can solve problems such as limitations in therapeutic use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: Determination of chlorine content in HA-Cl and HA conjugates
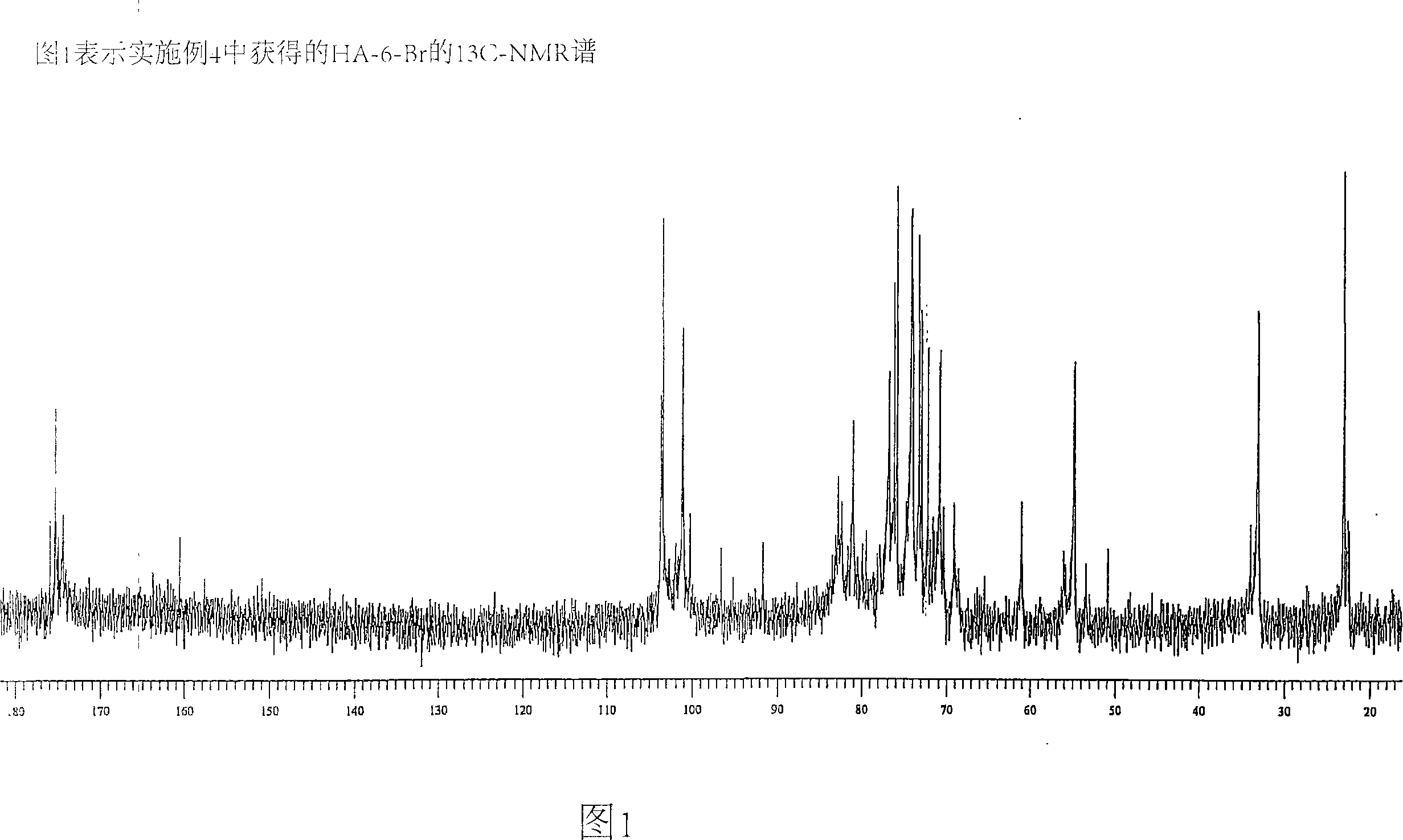
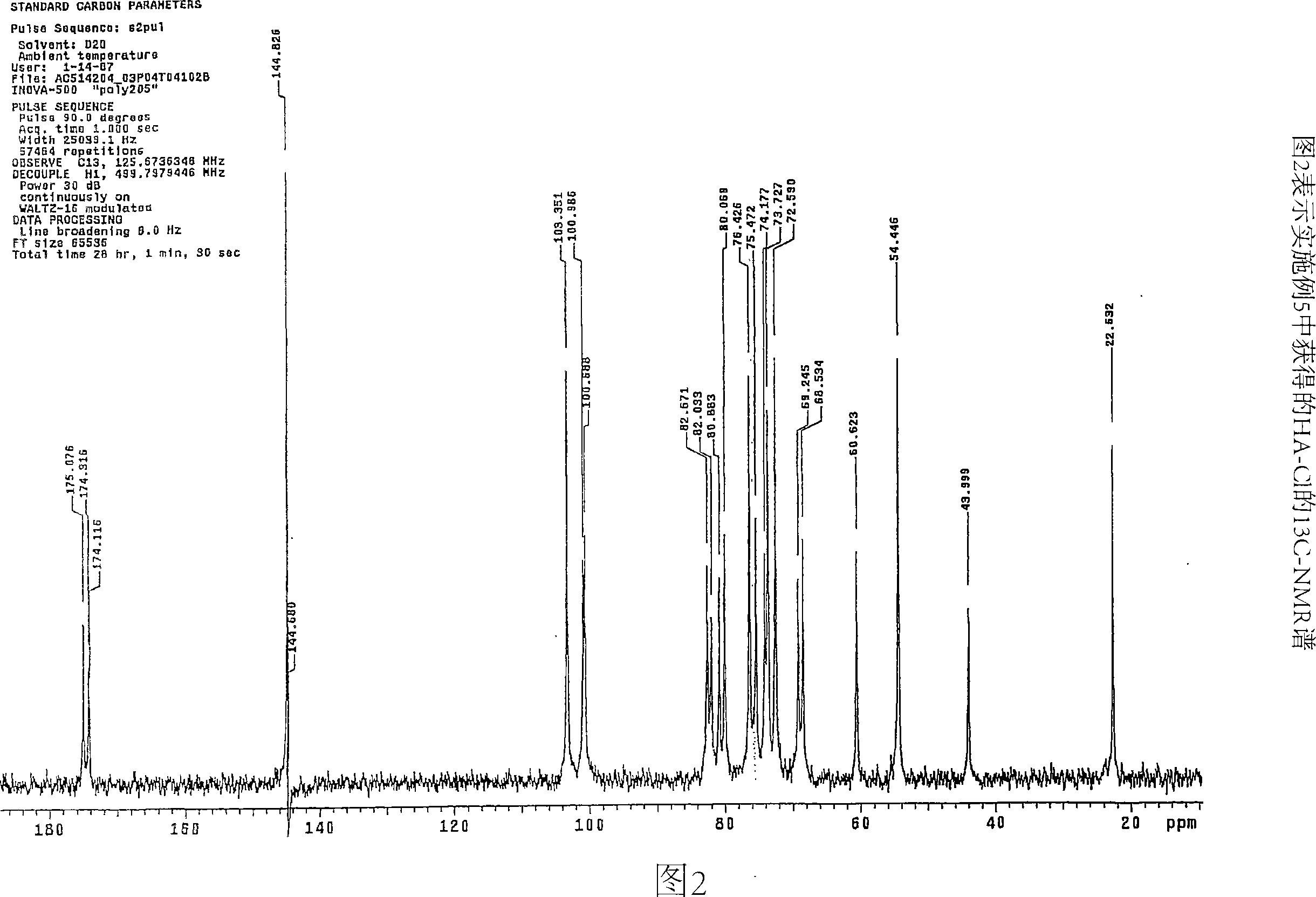
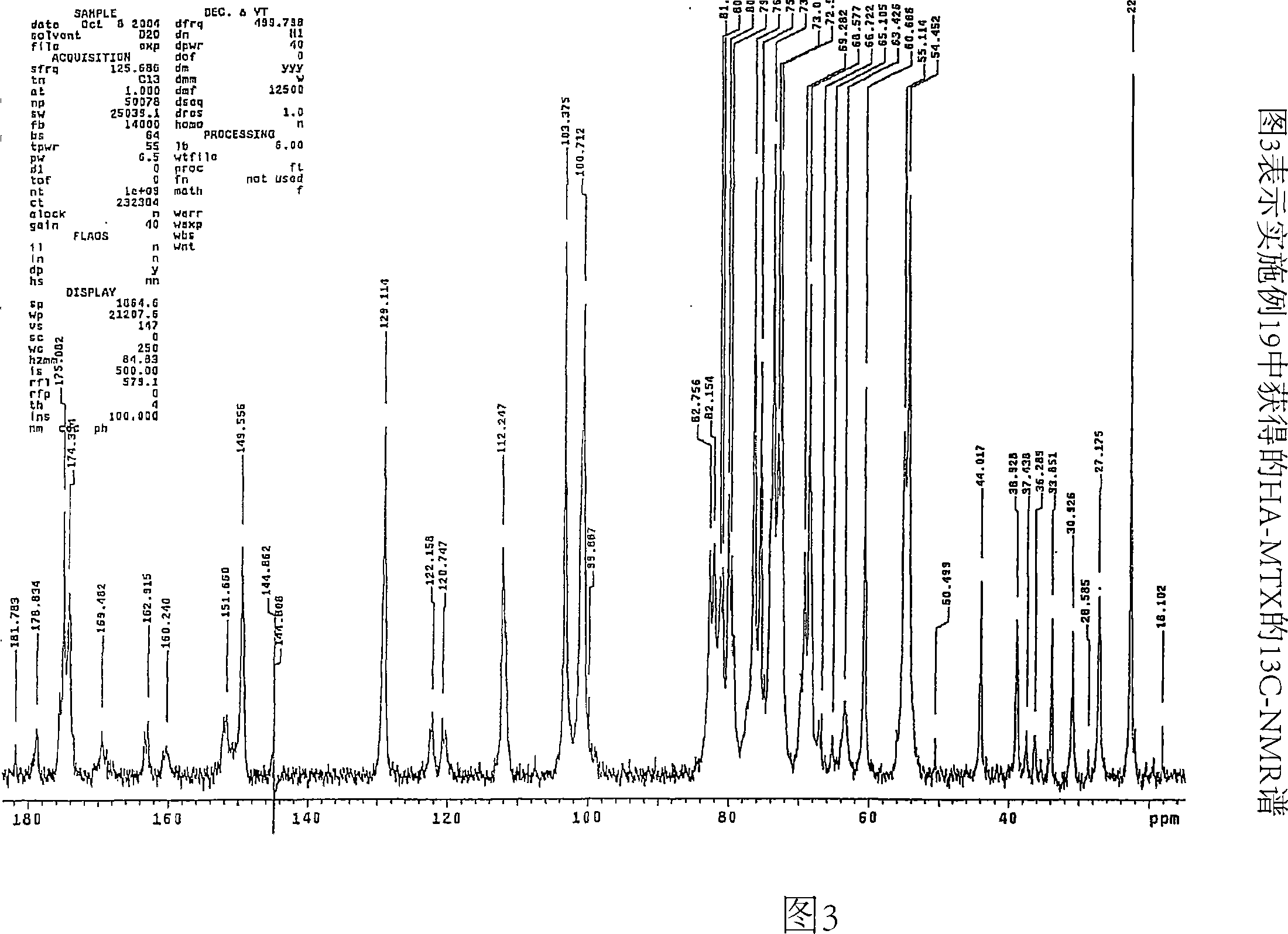
[0095] pass 13 CNMR (Spectrometer Varian Mercury 200), using 13 Standard sequence for C-spectrum capture (std 13 C sequence) to determine the chlorine content of the conjugate Dissolve 20 mg of the conjugate sample in 650 μL in a 5 mm tube at room temperature D 2 O middle. Gentle heating (50°C) was used to remove air bubbles in the solution. Spectra were collected after 24 hr at 30 °C and analyzed by integrating the CH of HA-Cl 2 OH signal (61ppm) and C h 2 Cl signal (44ppm) was analyzed. For the HA-MTX-Cl derivatives, in addition to these two signals, the C h 2 The OMTX signal (64ppm) is integrated. The chlorine content was determined as the ratio of the number of HA repeat units containing 6-chloro groups to the total number of HA repeat units. This ratio is then converted to weight percent chlorine.
Embodiment 2
[0096] Embodiment 2: determine methotrexate content by HPLC
[0097] The methotrexate content of the HA conjugates was determined by HPLC, analyzing samples before and after alkaline hydrolysis according to the accepted monograph on methotrexate (USP 23-p 984). The analysis conditions are, chromatograph: DionexDX-600. Column: Column Phenomenex Synergi 4μHydro-RP80, column size: 150×460mm, column particle size: 4μ, temperature: 40°C, eluent: 90% 0.2M disodium hydrogen phosphate / 0.1M citric acid (630:270), 10% CH 3 CN, isocratic conditions: 0.5 mL / min. Detector: diode array (range 200-780 nm), selected wavelength for quantification: 302 nm, injection volume: 25 μl, run time 30 minutes. The solution used for the determination of free methotrexate was prepared by directly dissolving HA-MTX in MilliQ water at an appropriate concentration. The total methotrexate content was measured after alkaline hydrolysis in NaOH 0.1M at room temperature for 2 hours. After neutralization wi...
Embodiment 3
[0099] Embodiment 3: measure weight-average molecular weight (Mw)
[0100] The molecular weight of the hyaluronic acid conjugate was measured by HP-SEC (High Performance Size Exclusion Chromatography). The analysis conditions are, chromatograph: HPLC pump980-PU (Jasco serial number B3901325), equipped with a Rheodyne 9125 syringe. Column: TSK PWxI (TosoBioscience) G6000+G5000+G3000 6, 10, 13μm particle size; temperature: 40°C Mobile phase: NaCl0.15M+0.01%NaN 3 Flow rate: 0.8mL / min. Detector: MALLS (WYATT DAWNEOS-WYATT, USA), λ=690nm, (dn / dc=0.167mL / g), UV spectrophotometer detector 875-UV (Jasco, serial number D3693916), λ=305nm, the refractive index of the interferometer refers to OPTILABREX (WYATT, USA); λ=690nm, sensitivity: 128x; temperature: 35°C; injection volume: 100 μl, run time 60 minutes HA-Cl and HA to be analyzed - A sample of MTX was prepared at a concentration of about 1.0 mg / ml in 0.9% NaCl and kept under stirring for 12 hours. Then, the solution was filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com