A recombinant method for production of an erythropoiesis stimulating protein
A technology of erythropoietin and red blood cells, which is applied in the field of erythropoietin, can solve the problems of short plasma half-life and limitation, and achieve the effect of reducing clearance time, increasing activity, restoring health status and quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of recombinant erythropoietic-stimulating protein (NESP)
[0040] The DNA sequence encoding the highly glycosylated form of human erythropoietin was obtained by de novo synthesis method. This approach will allow better codon optimization for the particular mammalian cell to be used. In addition, synthetic DNA is used for eukaryotic / prokaryotic expression purposes, providing isolatable quantities of polypeptides exhibiting the biological properties of naturally occurring erythropoietin (EPO), as well as the in vivo and in vitro biological activities of EPO.
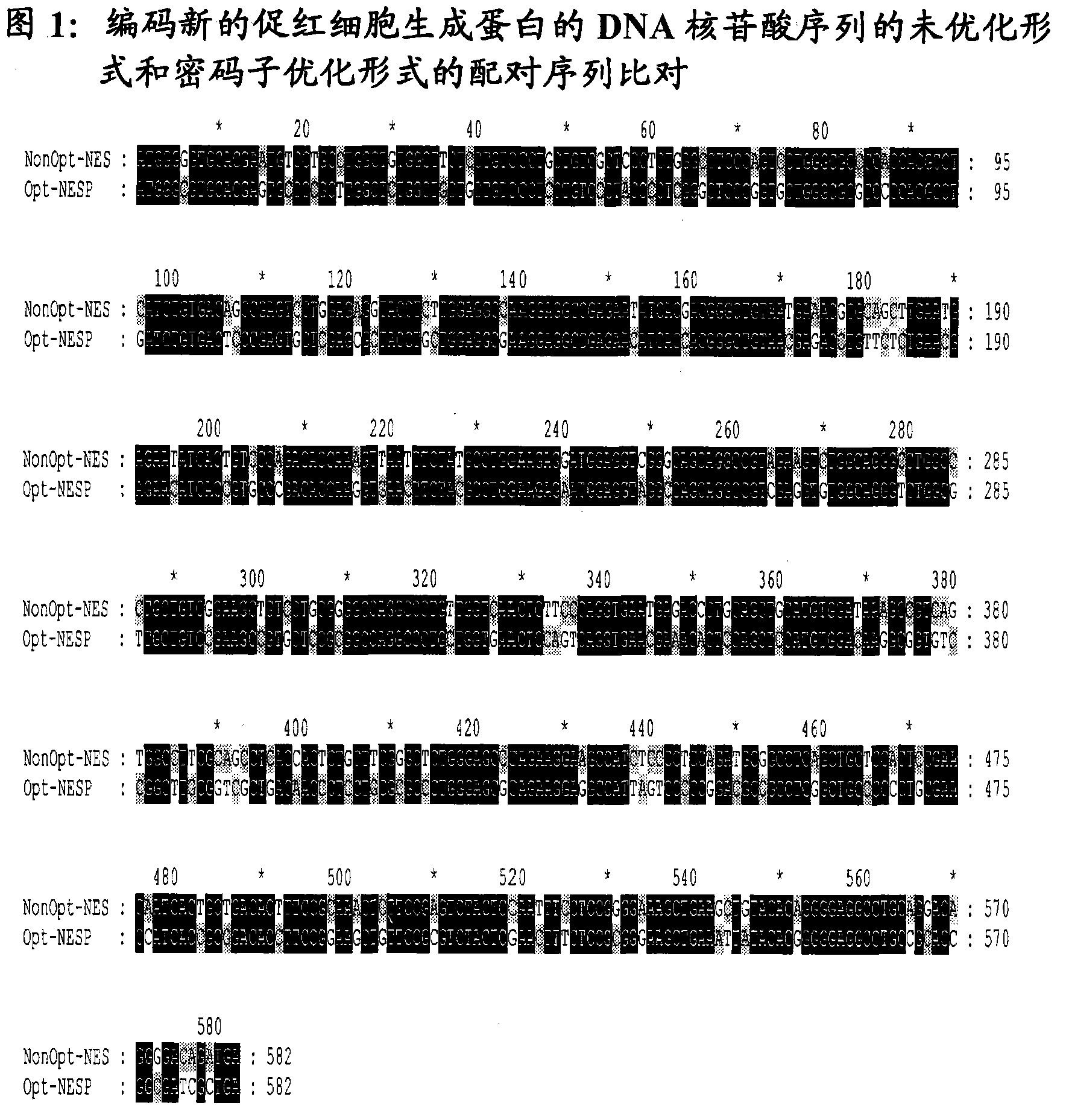
[0041]The nucleotide sequence encoding the erythropoietic-stimulating protein is shown in SEQ ID No.1. Nucleotide residues in the erythropoietin protein that are altered by incorporation of additional glycosylation sites compared to the naturally occurring transcript of the human gene encoding erythropoietin are highlighted in capital letters .
[0042] As part of the codon optimization proce...
Embodiment 2
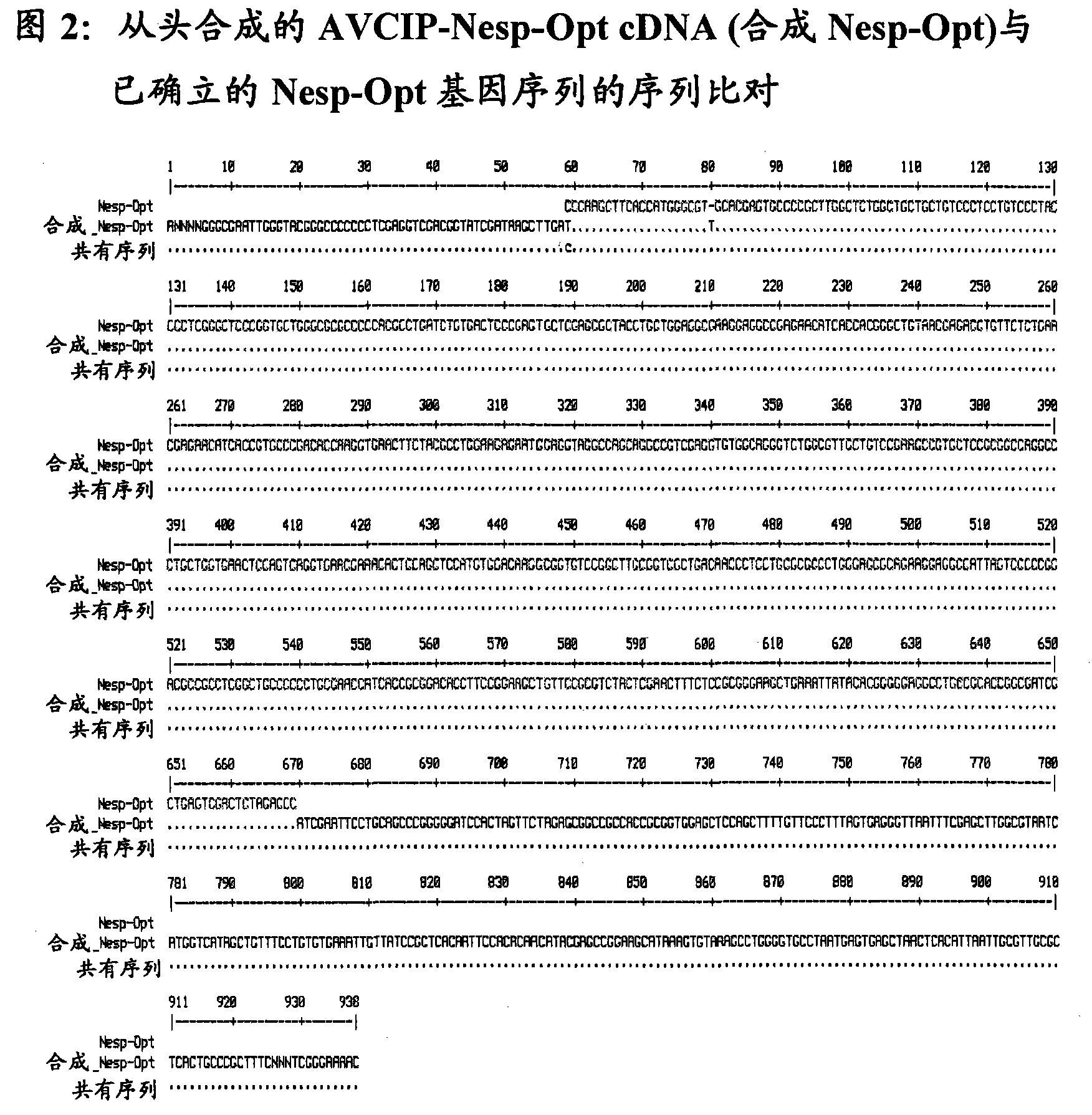
[0045] Example 2: Authenticity verification of de novo synthesized cDNA encoding erythropoietic proteins
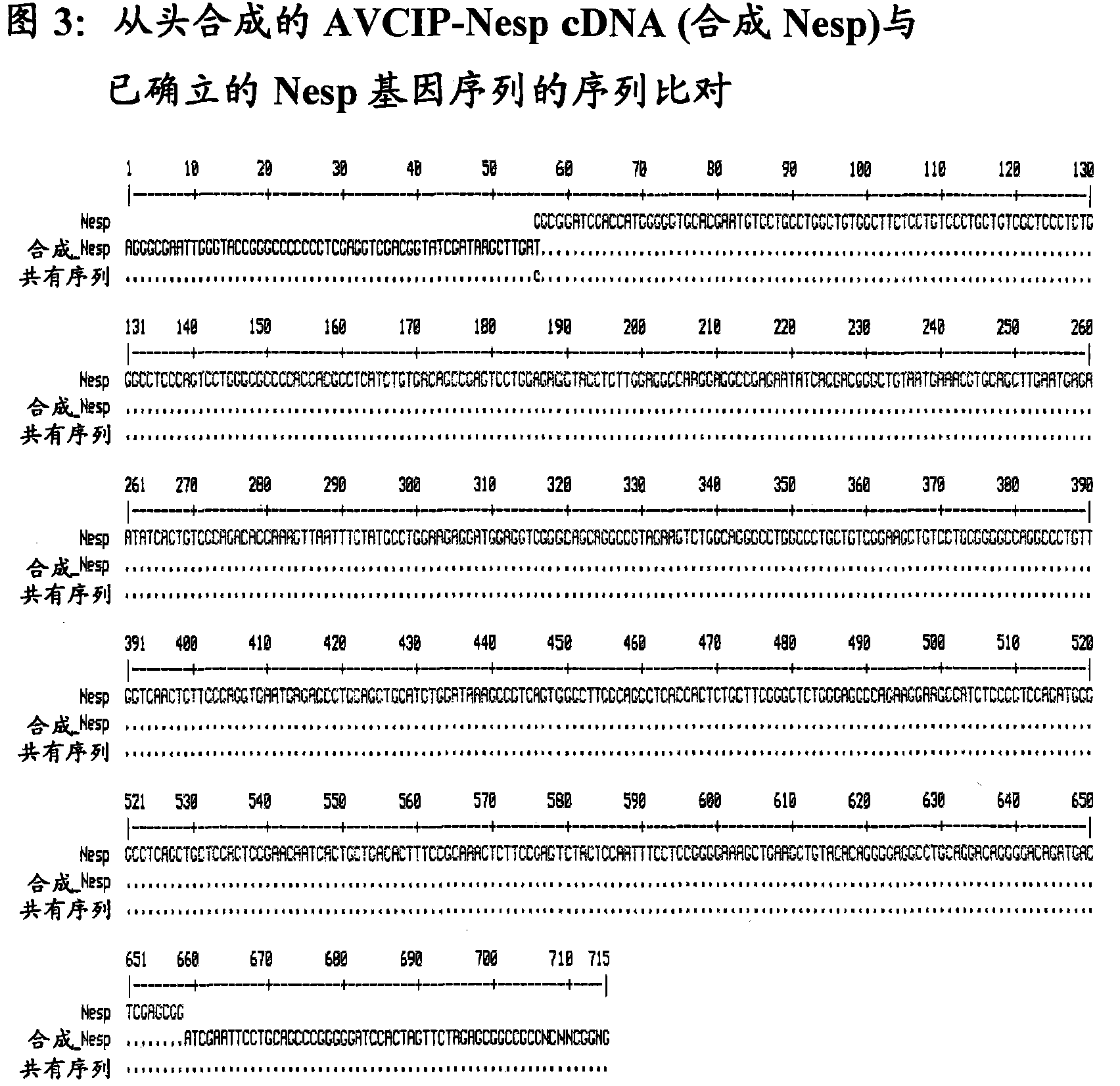
[0046] The authenticity of the original cDNA sequence (AVCIP-Nesp) and codon-optimized cDNA sequence (AVCIP-Nesp-Opt) synthesized from scratch was verified by automated DNA sequencing, and the results are shown in Figure 2 and Figure 3.
Embodiment 3
[0047] Example 3: Subcloning AVCIP-Nesp and AVCIP-Nesp-Opt cDNA into mammalian cell-specific expression vector pcDNA3.1D / V5-His
[0048] The de novo synthesized original cDNA sequence (AVCIP-Nesp) and codon-optimized cDNA sequence (AVCIP-Nesp-Opt) were subcloned into the mammalian cell-specific expression vector pcDNA3.1D / V5-His to produce ready-to-use Transfected constructs. Details of the method used are given below:
[0049] A. Reagents and Enzymes:
[0050] 1. QIAGEN Gel Extraction Kit and PCR Purification Kit
[0051] 2. pcDNA3.1D / V5-His vector DNA (Invitrogen)
[0052]
[0053] All reactions were performed according to the manufacturer's recommended procedures. For each reaction, dilute the provided 10x reaction buffer to a 1x final concentration.
[0054] B. Restriction Digestion of Vector and Insert:
[0055] method
[0056] Use the following DNA samples and restriction enzymes:
[0057]
[0058] Restriction enzyme digestion reaction:
[0059]
[0060...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com