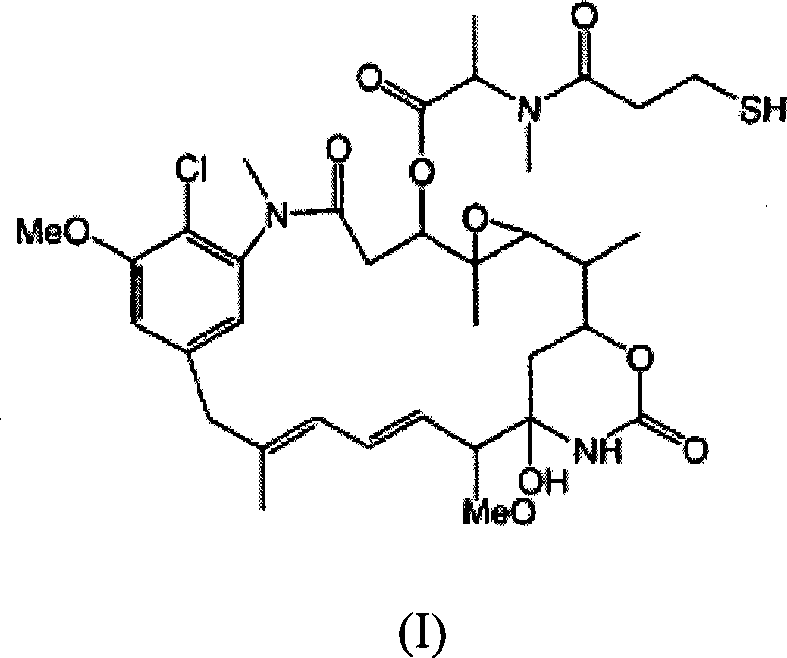
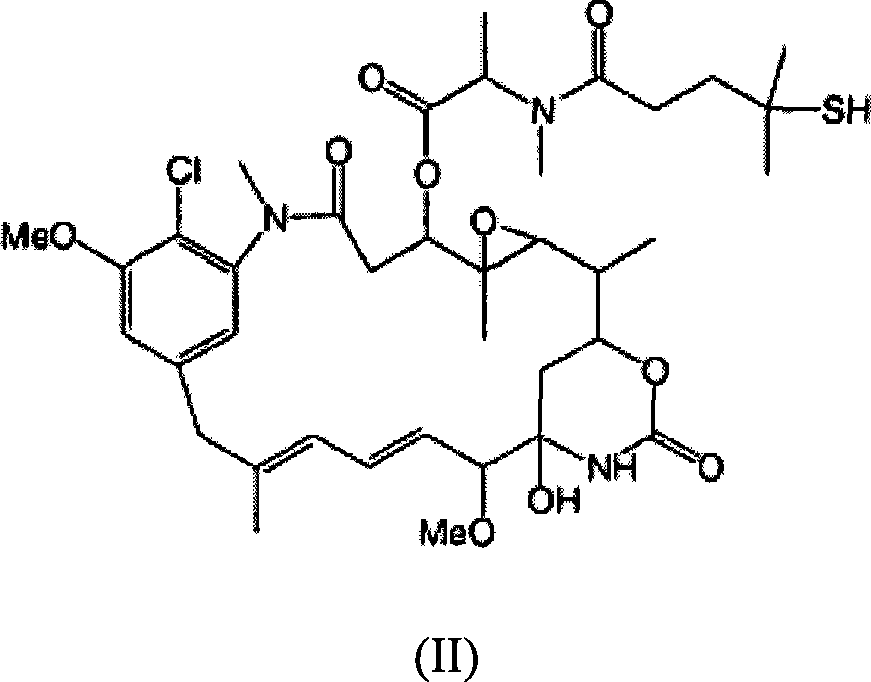
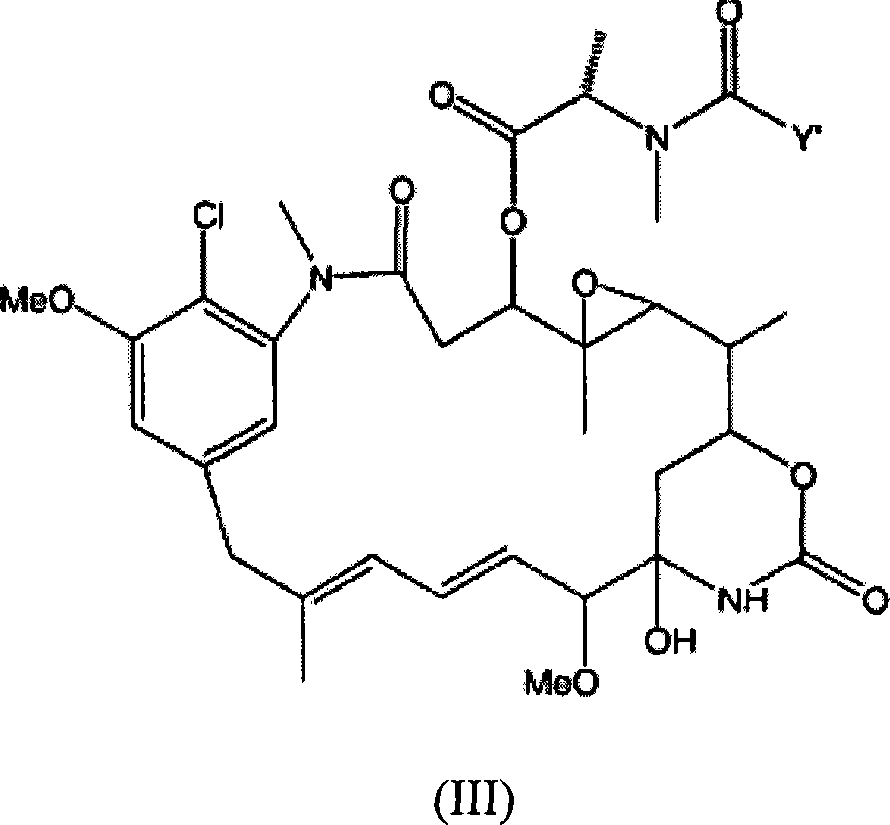
Immunoconjugate formulations
A technology for immunoconjugates and dosage forms, which can be used in immunoglobulins, antibody medical components, peptides, etc., to solve problems such as insufficient treatment of particles and aggregates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] This example shows the effect of the following dosage form excipients on the appearance of the formed MAb-DM1 conjugate sample.
[0150] A sample containing 1.0 mg / mL of huN901-SPP-DM1 in a phosphate buffer solution was prepared. The phosphate buffer solution was 10 mM, pH 6.5, 140 mM NaCl and each excipient listed in the following table. The excipient was directly added to the huN901-SPP-DM1 sample in units of w / w% (weight of excipient / weight of solution). Immediately after the addition of excipients, the dosage form mixture is filtered, and then the appearance and particle count tests are performed. The samples were then stored at 40°C for the duration of the study, and then tested again at 2 weeks and 1 month. As for the appearance, the sample is tested by checking the clarity of at least 1.0 mL of the solution on a white background and whether it has visible particles in white light relative to a black background. Record the results regarding the presence or absence of ...
Embodiment 2
[0168] This example shows the effect of amino acids on the stability of huN901-SPP-DM1 in terms of conjugate aggregates.
[0169] A 5.0 mg / mL huN901-SPP-DM1 sample was prepared in the following buffer, and then stored at 2-8°C and 25°C for 12 months. The content of conjugate aggregates was tested by chromatographic analysis at 1, 3, 6 and 12 months.
[0170] (1) 10mM sodium phosphate, 140mM NaCl, pH 6.5.
[0171] (2) 10mM sodium citrate, 135mM NaCl, 0.01% polysorbate 20, pH 5.5.
[0172] (3) 10 mM sodium citrate, 130 mM histidine, 0.01% polysorbate 20, pH 5.5.
[0173] (4) 10mM sodium citrate, 110mM glycine, 80mM NaCl, 0.01% polysorbate 20, pH 5.5.
[0174] 2-8℃
[0175] Example 1 shows that histidine improves the dosage form in terms of inhibiting the formation of coupling aggregates. Glycine also has this advantage.
Embodiment 3
[0177] This example shows the effect of histidine on the stability of the huMy9-6-SPDB-DM4 conjugate in terms of conjugate aggregates.
[0178] A 5.0 mg / mL huMy9-6-SPDB-DM4 conjugate was formed in the following solution:
[0179] (1) 10mM sodium citrate, 135mM NaCl, pH5.5
[0180] (2) 150mM histidine / histidine chloride, pH5.5
[0181] The samples were kept at 2-8°C and 25°C for 6 months, and then the aggregation of the conjugate was tested by chromatographic analysis.
[0182] Dosage form
[0183] The data shows that histidine has the beneficial effect of preventing aggregate formation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com