Diamine, polyamic acid and polyimide produced with the same
A technology of polyamic acid and phenylene, which is used in organic chemistry, liquid crystal materials, steroids, etc., can solve the problem of inability to make large changes, and achieve the effect of large pretilt angle and good alignment properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
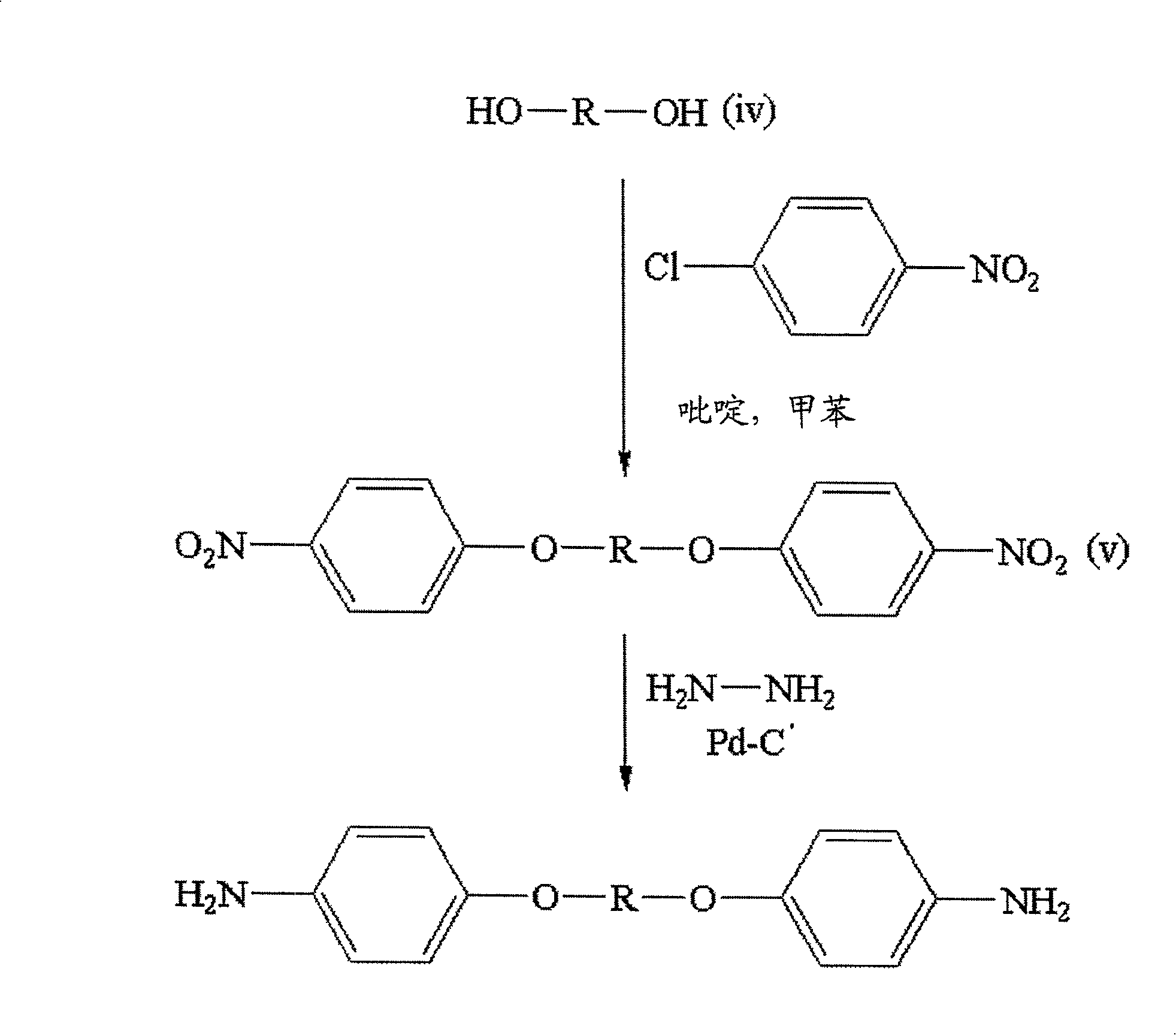
[0069] The diamine of the present invention can be prepared by a general diamine preparation method, for example, using acid or alcohol and nitrobenzene (such as 4-chloro-nitrobenzene) with a good leaving group (good leaving group, such as halogen) After the substitution reaction, followed by the reduction reaction in the system. The common preparation method of a specific embodiment of the diamine of the present invention is to react the following formula (i) and / or the following formula (ii) with the following formula (iii), and then selectively carry out a reduction reaction. have to:
[0070]
[0071] , in the above formulas (i) and (ii), R' and "are hydrogen or oxygen respectively, and G 1 and G 2 Respectively a group selected from: carboxyl (carboxyl), halogen, hydroxyl or amino group, in the formula (iii), X 1 and Y 1 are respectively selected from: carboxyl, halogen, hydroxyl or amino groups, and R is the range defined in the above formula (I). When R' and R " ...
Embodiment 1
[0094] [Example 1] Preparation of diamine
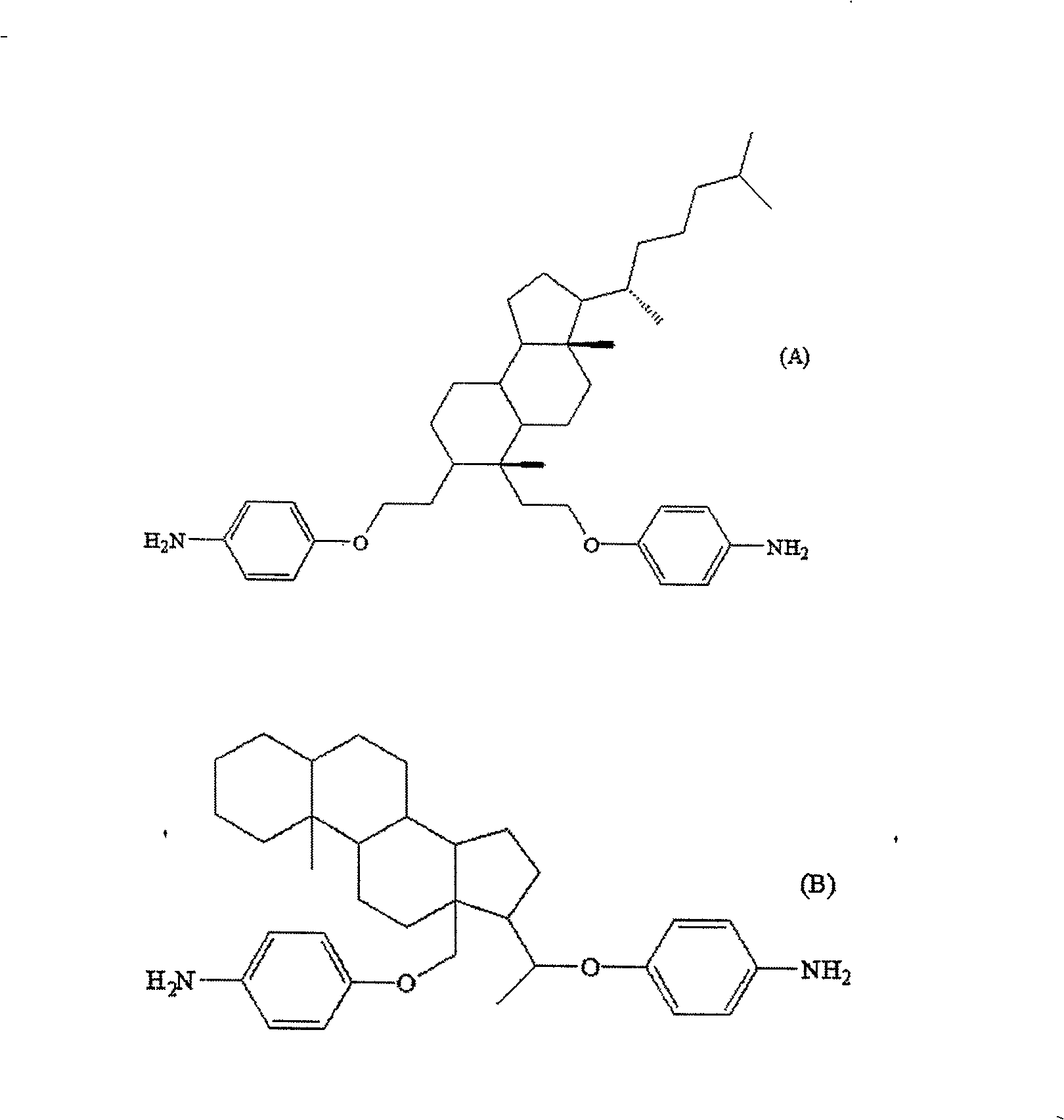
[0095] According to the following table 1, select the starting material (the compound shown in the formula (iv)) of each embodiment, and then according to the diamine production method in the above-mentioned common production method, respectively prepare the diamine of embodiment 1 (A) ~ (H). The diamines (A)~(H) of embodiment 1 are respectively as follows Figure 3-5 shown.
[0096] In order to verify the structure of the obtained diamine (E), a nuclear magnetic resonance spectrometer (manufactured by a German company, model Bruker Avance 600) was used for structural identification, and the obtained results were as follows:
[0097] Compound shown in formula (v) [wherein R is CH 2 -CH(C 14 h 29 )]:
[0098] 1 H-NMR (400MHz, CDCl 3 ), δ (ppm): 8.21 (dd, 4H); 6.99 (dd, 4H);
[0099] 4.80(t, 1H); 4.23(d, 2H); 1.85(t, 2H); 1.25(t, 20H); 0.88(d, 3H).
[0100] Diamine (E):
[0101] 1 H-NMR (400MH, CDCl 3 ), δ (ppm): 6.78 (dd,...
Embodiment 2
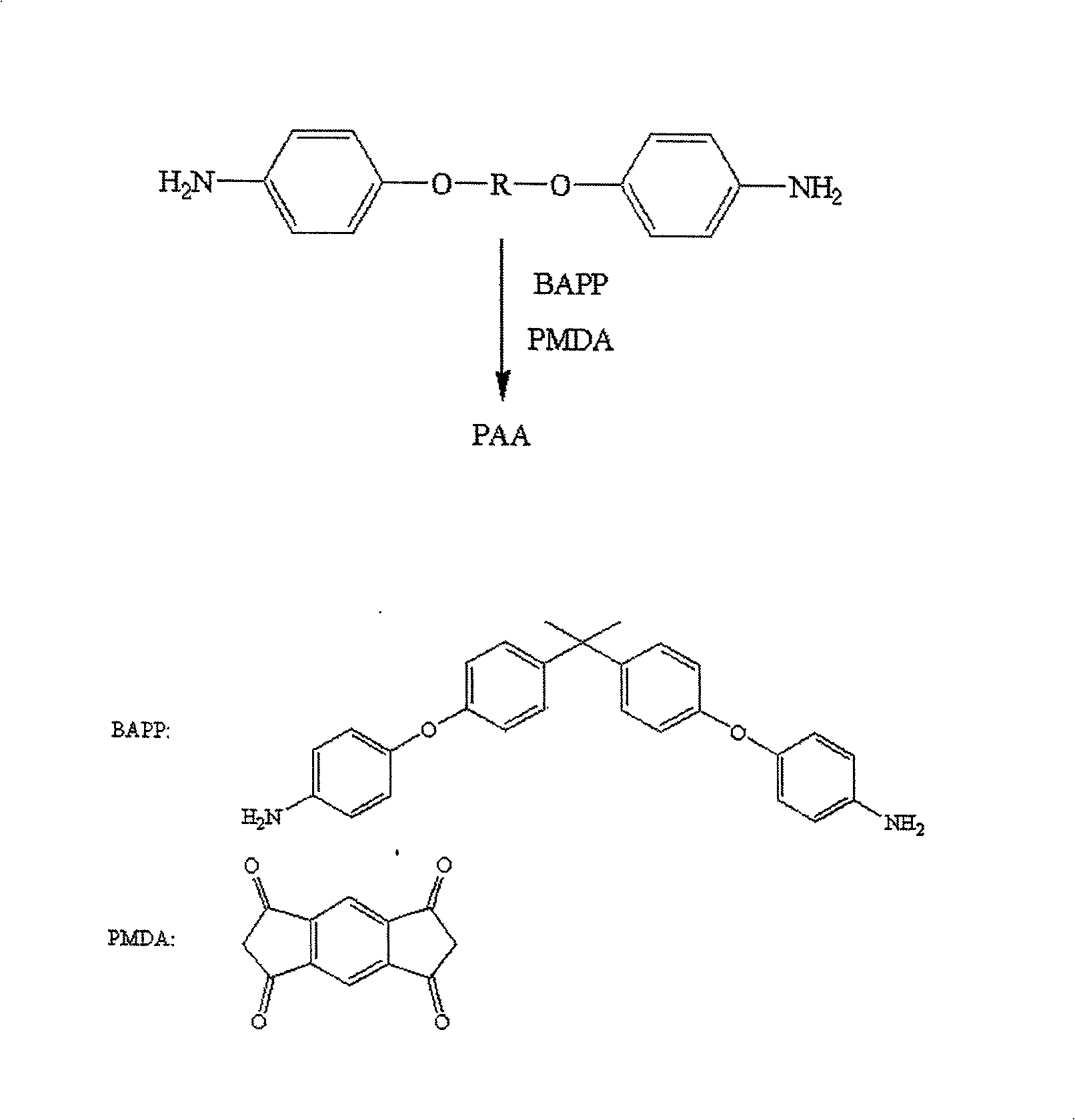
[0107] [embodiment 2] the preparation of polyamic acid and polyimide
[0108] According to the content ratio in Table 2 below, use the above-mentioned diamine (A), diamine (E) or BAPP as the diamine component, and use PMDA as the dianhydride, and then use the polyamic acid and The preparation method of polyimide can be prepared respectively to obtain the polyimide layers numbered 2-1 to 2-9 formed on the ITO glass.
[0109] test:
[0110] According to the above-mentioned sample production process and the ITO glass with the polyimide layer formed on the above-mentioned numbers 2-1 to 2-9 were used for sample production, and the samples numbered 2-1 to 2-9 were respectively obtained, Then, tests were carried out using the above-mentioned pretilt angle measurement and alignment property observation methods, and the results are summarized in Table 2 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com